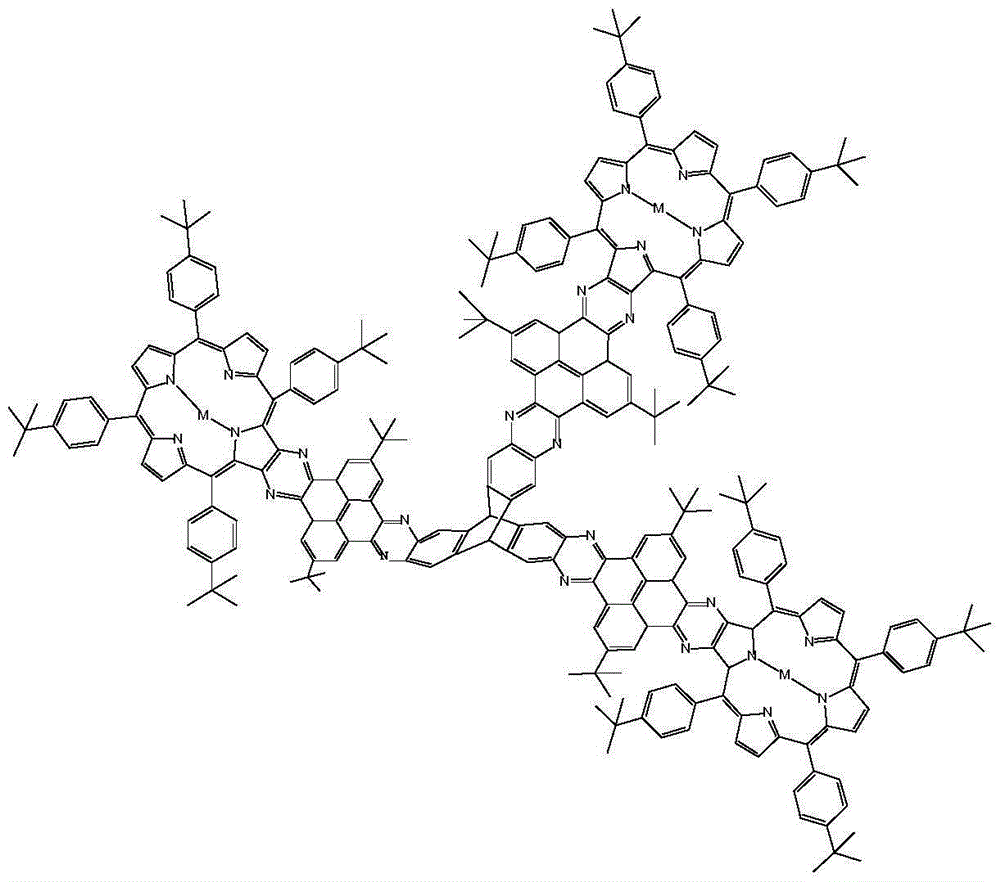
Serial compounds with triptycene as framework and in bridge connection with metalloporphyrin through pyrene tetrone and preparation method therefor
A technology of metalloporphyrin and pyrenetetrane bridge, which is applied in the field of photoelectric conversion materials, can solve the problems of general poor solubility, multiple by-products, difficult separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation method of pyrenetetraone bridged triptycene-copper porphyrin comprises the steps:
[0073] (1) Synthesis of 2,6,14-trinitrotriptycene
[0074] Add 200ml of concentrated nitric acid and 5g of triptycene to a 500mL round bottom flask in turn, stir, heat to 65°C, reflux for 12-24h under stirring, add water and stir after cooling to room temperature, filter the precipitate with a Buchner funnel, and wash with water for two time, vacuum-dried to obtain a pale yellow solid; use 200-300 mesh silica gel to pass through the column, the eluent is ethyl acetate:petroleum ether=1:3, collect the product of the third band, and obtain 2,6,14-trinitro Triptycene compound 0.8g, yield 33%.
[0075] (2) Synthesis of 2,6,14-triaminotriptycene
[0076] Add 50ml of dichloride, 0.8g of 2,6,14-trinitrotriptycene, 1.65g of sodium borohydride and 0.2g of palladium / carbon (5%) to a 250ml pear-shaped bottle, and react for 1 to 2 hours under nitrogen protection , add 10ml of meth...
Embodiment 2
[0099] The preparation method of pyrenetetraone bridged triptycene-nickel porphyrin compound comprises the steps:
[0100] (1) Synthesis of 2,6,14-trinitrotriptycene
[0101] Add 200ml of concentrated nitric acid and 5g of triptycene to a 500mL round bottom flask in turn, stir, heat to 65°C, reflux for 12-24h under stirring, add water and stir after cooling to room temperature, filter the precipitate with a Buchner funnel, and wash with water for two time, vacuum-dried to obtain a pale yellow solid; use 200-300 mesh silica gel to pass through the column, the eluent is ethyl acetate:petroleum ether=1:3, collect the product of the third band, and obtain 2,6,14-trinitro Triptycene compound 0.8g, yield 33%.
[0102] (2) Synthesis of 2,6,14-triaminotriptycene
[0103] Add 50ml of dichloride, 0.8g of 2,6,14-trinitrotriptycene, 1.65g of sodium borohydride and 0.2g of palladium / carbon (5%) to a 250ml pear-shaped bottle, and react for 1 to 2 hours under nitrogen protection , add 10m...
Embodiment 3
[0119] The preparation method of pyrenetetraone bridged triptycene-palladium porphyrin compound comprises the steps:
[0120] (1) Synthesis of 2,6,14-trinitrotriptycene
[0121] Add 200ml of concentrated nitric acid and 5g of triptycene to a 500mL round bottom flask in turn, stir, heat to 65°C, reflux for 12-24h under stirring, add water and stir after cooling to room temperature, filter the precipitate with a Buchner funnel, and wash with water for two time, vacuum-dried to obtain a pale yellow solid; use 200-300 mesh silica gel to pass through the column, the eluent is ethyl acetate:petroleum ether=1:3, collect the product of the third band, and obtain 2,6,14-trinitro Triptycene compound 0.8g, yield 33%.
[0122] (2) Synthesis of 2,6,14-triaminotriptycene
[0123]Add 50ml of dichloride, 0.8g of 2,6,14-trinitrotriptycene, 1.65g of sodium borohydride and 0.2g of palladium / carbon (5%) to a 250ml pear-shaped bottle, and react for 1 to 2 hours under nitrogen protection , add 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com