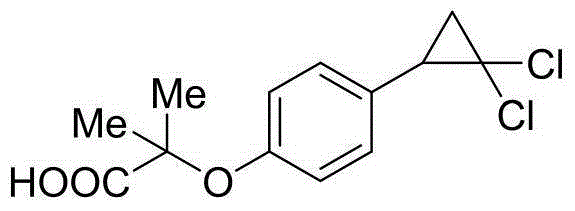
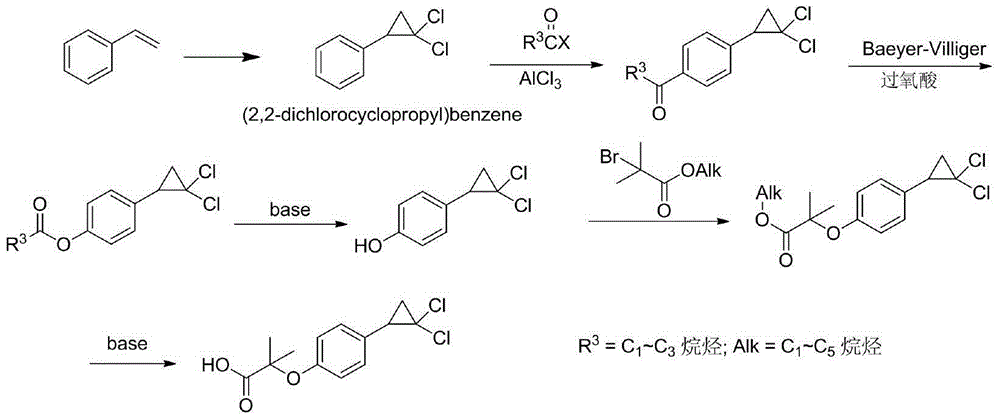
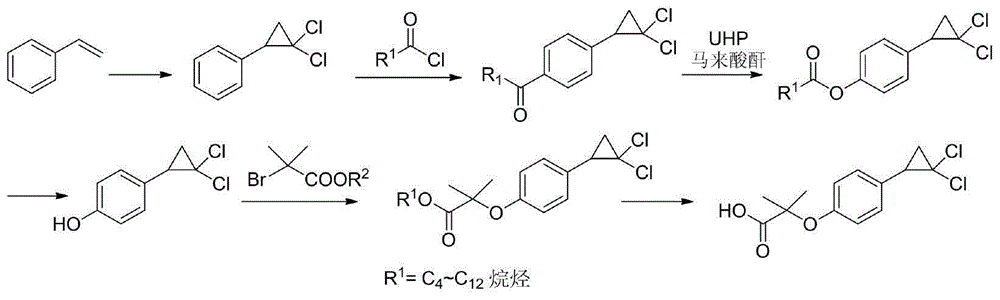
Method for preparing hypolipidemic medicine ciprofibrate with p-coumaric acid
A technology for p-coumaric acid and ciprofibrate, which is applied in the synthesis field of the hypolipidemic drug ciprofibrate, can solve problems such as serious environmental pollution, and achieves the effects of safe operation, environmental friendliness, and ease of large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for preparing the blood lipid-lowering drug ciprofibrate by using p-coumaric acid, using p-coumaric acid as a starting material to obtain ciprofibrate through the processes of decarboxylation, etherification, cyclization, and alcoholysis, the specific steps include:
[0051] (1) Preparation of p-hydroxystyrene (Ⅱ)
[0052] Add 150 g of p-coumaric acid, 750 mL of DMF, and 9 g of potassium acetate into a 1 L reaction flask, and heat to 150° C. for 2-3 h (monitored by TLC). After the reaction was complete, the reaction solution was cooled down to room temperature, and concentrated to dry DMF under reduced pressure. Add 200mL ethyl acetate and 100mL water to the residue, stir for 15min, separate the liquids, extract the water phase once more with 100mL ethyl acetate, combine the organic phases, wash the organic phase once with 100mL2mol / L sodium carbonate aqueous solution solvent, wash once with 100mL water Wash once, and finally wash once with 100mL saturated br...
Embodiment 2
[0060] A method for preparing the blood lipid-lowering drug ciprofibrate by using p-coumaric acid, using p-coumaric acid as a starting material to obtain ciprofibrate through the processes of decarboxylation, etherification, cyclization, and alcoholysis, the specific steps include:
[0061] (1) Preparation of p-hydroxystyrene (Ⅱ)
[0062] Add 150g of p-coumaric acid, 550mL of DMA, and 9g of sodium acetate into a 1L reaction flask, and heat to 150°C for 2-3h (monitored by TLC). After the reaction was complete, the reaction liquid was lowered to room temperature, and concentrated under reduced pressure to dry DMA. Add 200mL ethyl acetate and 100mL water to the residue, stir for 15min, separate the liquids, extract the water phase once more with 100mL ethyl acetate, combine the organic phases, wash the organic phase once with 100mL2mol / L sodium carbonate aqueous solution solvent, wash once with 100mL water Wash once, and finally wash once with 100mL saturated brine, dry over an...
Embodiment 3
[0070] A method for preparing the blood lipid-lowering drug ciprofibrate by using p-coumaric acid, using p-coumaric acid as a starting material to obtain ciprofibrate through the processes of decarboxylation, etherification, cyclization, and alcoholysis, the specific steps include:
[0071] (1) Preparation of p-hydroxystyrene (Ⅱ)
[0072] Dissolve 150Kg of p-coumaric acid in 710Kg of DMF, pump the mixture into a 1000L stainless steel reaction axe, start mechanical stirring, and then add 9Kg of potassium acetate. Steam was heated to 150°C for 2-3h (monitored by HPLC). After the reaction is complete, turn off the steam, pass circulating water down to room temperature, and recover DMF under reduced pressure. Add 180Kg ethyl acetate and 100Kg water to the concentrated ash under stirring, stir for 30min, separate the liquids, extract the water phase with 90Kg ethyl acetate once, combine the organic phases, and wash the organic phase once with 100Kg2mol / L sodium carbonate aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com