Medicine urapidil composition capsule for treating hypertensive crisis of old people
A technology of urapidil and its composition, which is applied in the field of urapidil composition capsules for the treatment of elderly hypertensive crisis, which can solve the problems of increasing the risk of medication for patients, the toxicity of patients, and poor stability, and is suitable for clinical application , high bioavailability, improved fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of urapidil crystals
[0030] Get urapidil crude drug, add in the mixed solvent A of acetone, N-methylacetamide that the volume of 40 ℃ is 8 times of urapidil weight, acetone, N-methylacetamide volume ratio is 4:1.5, obtains solution; then applying a constant magnetic field with a magnetic field intensity of 0.5T in the horizontal direction of the liquid surface of the resulting solution, and under the condition of this constant magnetic field, in the solution, the volume is added dropwise to isobutanol and ether that are 5 times the weight of urapidil The volume ratio of the mixed solvent B, isobutanol and diethyl ether is 4:3; after the dropwise addition, the temperature was lowered to -5°C, allowed to stand for 2 hours, filtered, washed, and vacuum-dried to obtain the urapidil crystals.
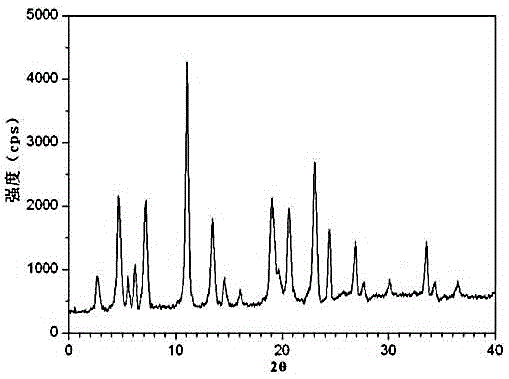
[0031] The X-ray powder diffraction pattern obtained by measuring the obtained urapidil crystal using Cu-Kα ray is as follows: figure 1 shown.
Embodiment 2
[0032] Example 2: Preparation of Urapidil Capsules
[0033] Prescription: in parts by weight
[0034]
[0035] Preparation:
[0036] (1) Processing of raw and auxiliary materials: crush urapidil to 100 meshes;
[0037] (2) Weighing: Weighing according to the process prescription;
[0038] (3) Preparation of adhesive: dissolve povidone K30 in 95% ethanol and set aside;
[0039] (4) Mixing and granulation: Add urapidil, microcrystalline cellulose, and nicotinamide to the wet mixing granulator, turn on the stirring motor and dry mix for 10 minutes; add the prepared binder to wet mix and cut, and use 24 Mesh sieve soft material;
[0040] (5) Drying: Add the wet granules obtained from granulation into a fluidized bed dryer, set the temperature at 65-70°C, and dry for 150-180 minutes. After drying, the material is granulated at 24 mesh;
[0041] (6) Mixing: Put the granulated particles and micropowder silica gel into the three-dimensional motion mixer, set the pre-mixing s...
Embodiment 3
[0044] Example 3: Preparation of Urapidil Capsules
[0045] Prescription: in parts by weight
[0046]
[0047] Preparation:
[0048] (1) Processing of raw and auxiliary materials: crush urapidil to 100 meshes;
[0049] (2) Weighing: Weighing according to the process prescription;
[0050] (3) Preparation of adhesive: dissolve povidone K30 in 95% ethanol and set aside;
[0051] (4) Mixing and granulation: Add urapidil, microcrystalline cellulose, and nicotinamide to the wet mixing granulator, turn on the stirring motor and dry mix for 10 minutes; add the prepared binder to wet mix and cut, and use 24 Mesh sieve soft material;
[0052] (5) Drying: Add the wet granules obtained from granulation into a fluidized bed dryer, set the temperature at 65-70°C, and dry for 150-180 minutes. After drying, the material is granulated at 24 mesh;
[0053] (6) Mixing: Put the granulated particles and micropowder silica gel into the three-dimensional motion mixer, set the pre-mixing s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com