A kind of preparation method of lithium nickel manganese oxide cathode material
A cathode material, lithium nickel manganese oxide technology, which is applied in the field of preparation of lithium nickel manganate cathode material, can solve the problems of difficult industrialized scale production, slow reaction speed and high cost, and achieves improved discharge specific capacity, increased intercalation amount, high specific energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
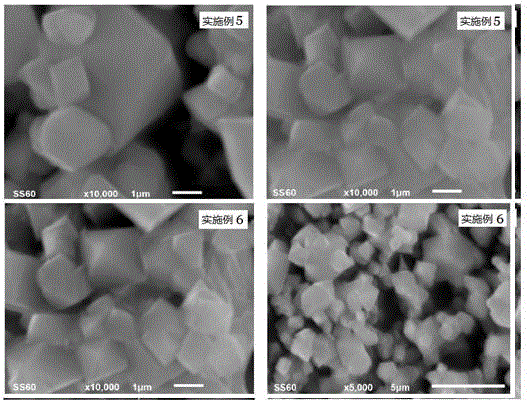
Examples
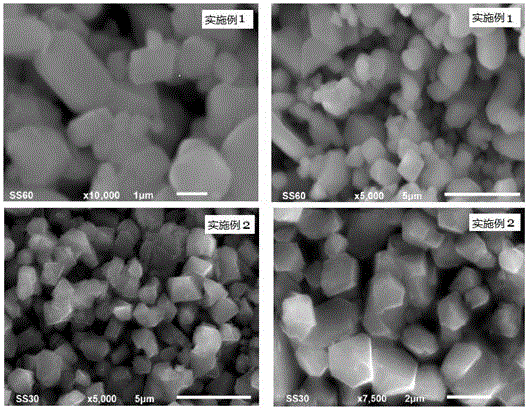
Embodiment 1
[0059] A preparation method of lithium nickel manganese oxide positive electrode material, the steps are:
[0060] The first step mixes and grinds 0.02mol of nickel nitrate, 0.06mol of manganese nitrate and 0.65mol of lithium acetate to obtain a mixture of nickel, manganese and lithium;
[0061] In the second step, the potassium persulfate of 0.084mol (1.05 times of the total molar amount of nickel salt and manganese salt in the first step) is mixed and ground with the mixture described in the first step to obtain a reaction mixture;
[0062] The third step is to transfer the reaction mixture described in the second step into a polytetrafluoroethylene reactor, add 1mL of distilled water, cover and seal with a stainless steel reactor jacket, control the reaction temperature at 60°C, and keep it warm for 36 hours to obtain the reactant;
[0063] The fourth step is to take out the reactant obtained in the third step, wash with distilled water until no sulfate is detected, and fil...
Embodiment 2
[0067] A preparation method of lithium nickel manganese oxide positive electrode material, the steps are:
[0068] The first step is the nickel chloride (0.021mol), nickel nitrate (0.021mol) that the equimolar ratio mixing of nickel molar total amount is 0.042mol, the manganese chloride (0.12mol equimolar ratio mixing with manganese molar total amount) 0.05mol), manganese nitrate (0.05mol), lithium hydroxide of 1.125mol are mixed and ground evenly, obtain nickel, manganese, lithium mixture;
[0069] In the second step, the ammonium persulfate of 0.168mol (1.05 times the total molar amount of nickel salt and manganese salt in the first step) is mixed and ground with the mixture described in the first step to obtain a reaction mixture;
[0070] The third step is to transfer the reaction mixture described in the second step into a polytetrafluoroethylene reactor, add 2 mL of deionized water, cover and seal with a stainless steel reactor jacket, control the reaction temperature at...
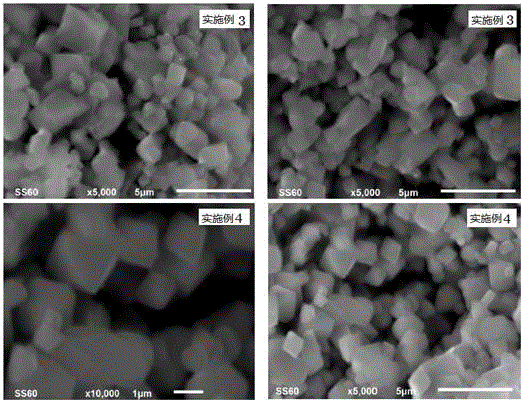
Embodiment 3
[0075] A preparation method of lithium nickel manganese oxide positive electrode material, the steps are:
[0076] In the first step, 0.06mol of nickel sulfate, mixed with 0.18mol of manganese sulfate and 2.05mol of lithium hydroxide are uniformly ground to obtain a mixture of nickel, manganese and lithium;
[0077] In the second step, the ammonium persulfate of 0.264mol (1.1 times of the total molar amount of nickel salt and manganese salt in the first step) is mixed and ground with the mixture described in the first step to obtain a reaction mixture;
[0078] The third step is to transfer the reaction mixture described in the second step into a polytetrafluoroethylene reactor, add 5mL of pure water, cover and seal the stainless steel reactor jacket, control the reaction temperature at 100°C, and keep it warm for 28 hours. The obtained reactant ;
[0079] The fourth step is to take out the reactant obtained in the third step, wash it with pure water until no sulfate is detec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com