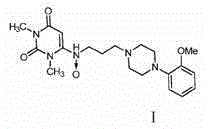
Anti-hypertension urapidil medicine compound
A technology of urapidil and anti-hypertension, applied in the field of medicine, can solve the problems of patients with toxicity, affect the quality of drugs, and poor stability, and achieve the effect of improving fluidity, improving drug efficacy, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
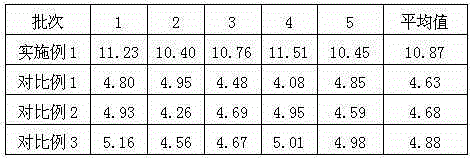
Embodiment 1
[0022] Embodiment 1: Preparation of urapidil crystal compound
[0023] (1) Add the crude urapidil to a mixed solution of chloroform and propanol whose volume is 4 times the weight of urapidil, the volume ratio of chloroform and propanol is 3:1, heat up to 30°C, and stir until completely dissolved;
[0024] (2) In a sound field with a frequency of 30KHz and an output power of 45W, add a mixed solution of ether, acetone, and cyclohexane whose volume is 8 times the weight of urapidil while stirring, and the volume ratio of ether, acetone, and cyclohexane It is 4:1:1, the stirring speed is 150 rpm, and the adding speed is 100 ml / min;
[0025] (3) After adding the mixed solution of ether, acetone, and cyclohexane, under a sound field with a frequency of 25KHz and an output power of 40W, cool down to -10°C at 10°C / hour, grow crystals for 2 hours, wash, and dry in vacuum , to obtain urapidil compound.
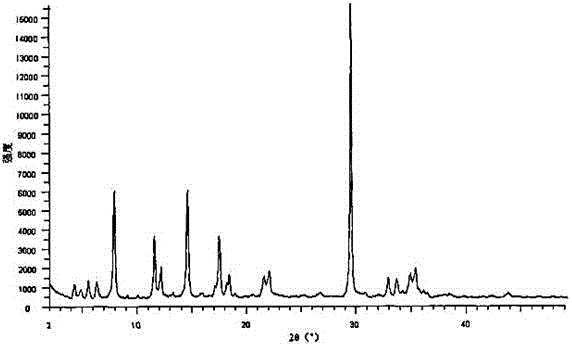
[0026] The X-ray powder diffraction pattern obtained by measuring the compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com