Amphiphilic derivatives of 3-((2-(dimethylamino)ethyl)(methyl)amino)propionic acid and uses thereof
A technology based on dimethylamino and ethyl groups, which is applied in the field of gene therapy, can solve the problems of complex preparation process and difficulty in scale-up production, and achieve the effects of increasing loading capacity, effective delivery, and increasing in vivo stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
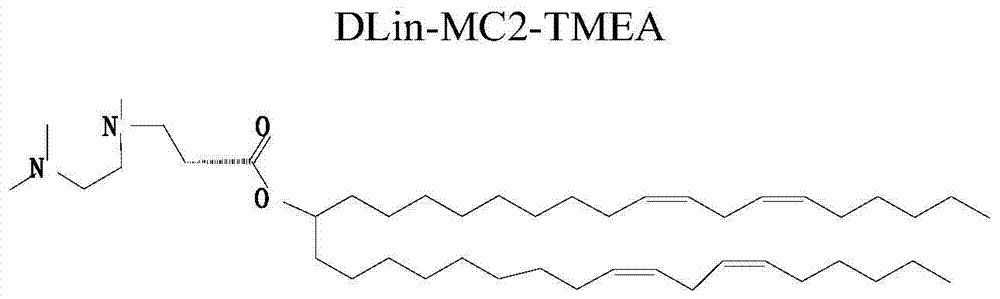
[0071] Example 1, 3-((2-(dimethylamino) ethyl) (methyl) amino) propionic acid amphiphilic derivative TMEA
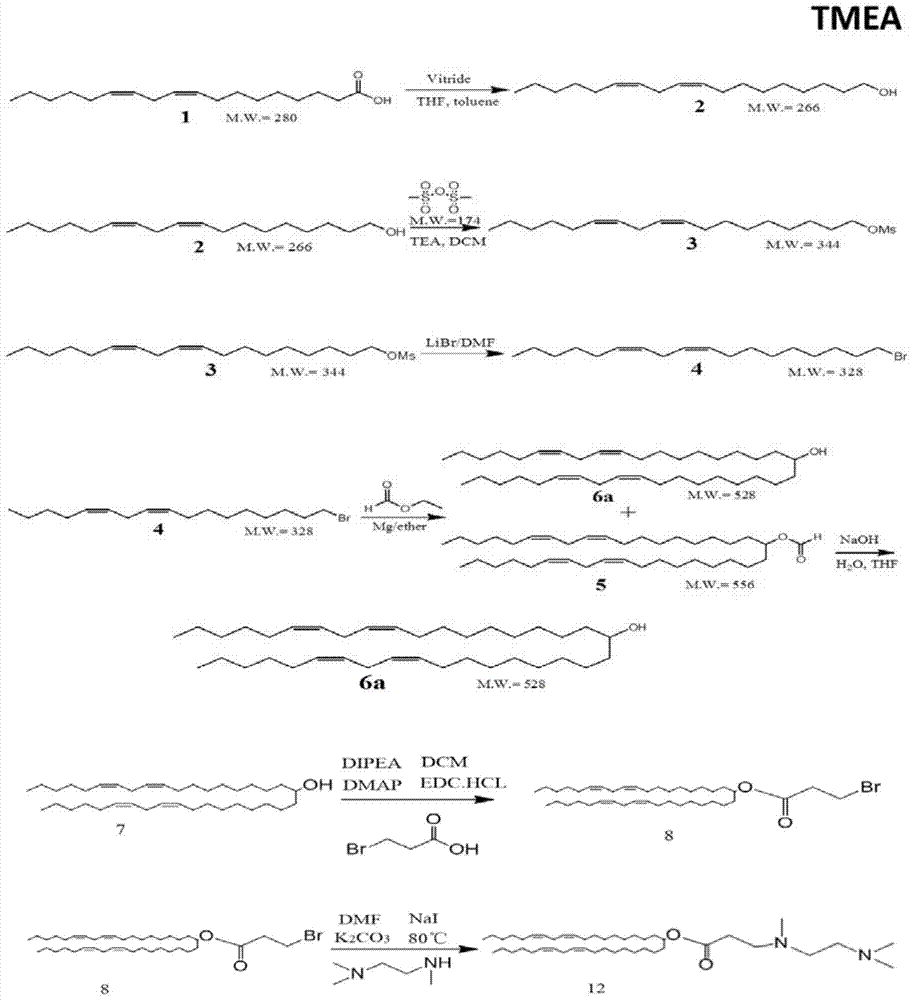
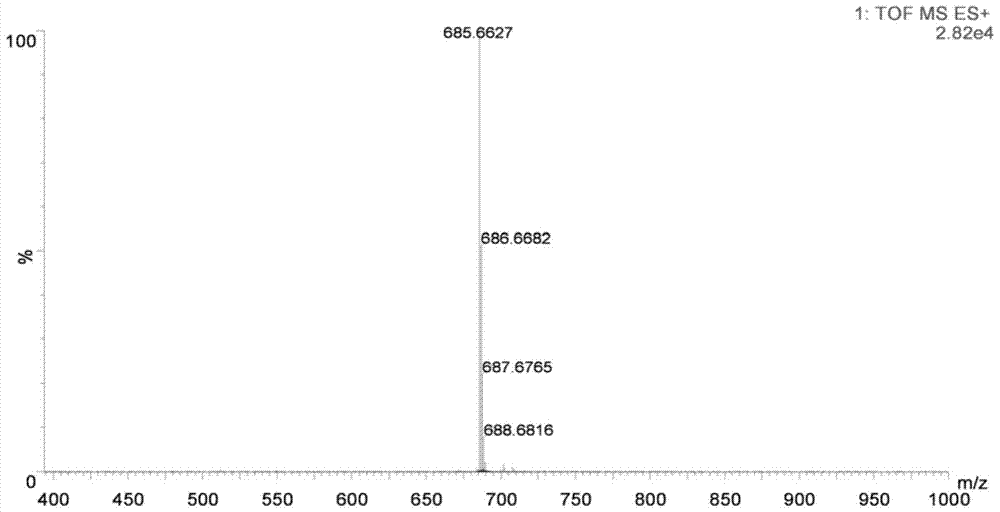
[0072] figure 1 It is the structural diagram of the amphiphilic compound TMEA; its preparation is as follows figure 2 As shown, specifically: choose N, N, N'-ethylenediamine, linoleic acid, etc. as raw materials, according to the reaction process figure 2 Synthesized to obtain TMEA lipids. image 3 NMR spectrum results for the amphiphilic compound TMAEA, Figure 4 Its mass spectrum results.
[0073] Reaction materials:
[0074] Linoleic acid, tetrahydrofuran (THF), red aluminum solution (vitride), toluene (toluene), anhydrous sodium sulfate, ethyl acetate, pure water, dichloromethane, triethylamine, DMAP (4-dimethylaminopyridine) , EDC·HCl (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride), methanesulfonic anhydride, PMA (propylene glycol methyl ether acetate), hydrochloric acid, sulfuric acid, chlorination Sodium, DMF (dimethylformamide), lithium br...
Embodiment 2
[0097] Example 2, 3-((2-(dimethylamino) ethyl) (methyl) amino) propionic acid amphiphilic derivative T1
[0098] Figure 5 It is the structural diagram of 3-((2-(dimethylamino)ethyl)(methyl)amino)propionic acid amphiphilic derivative T1; its preparation is as follows: select N, N, N'-ethylenediamine , palmitic acid, etc. as raw materials, synthesized according to the synthesis method of Example 1, the difference is: in step 1, reactant 1 is palmitic acid, and other synthesis steps, synthesis principles and raw materials are exactly the same. T1 lipids were obtained; all were purified by HPLC and identified by mass spectrometry, the purity was greater than 95%, and the molecular weight was consistent with the theoretical value.
Embodiment 3
[0099] Example 3, 3-((2-(dimethylamino) ethyl) (methyl) amino) propionic acid amphiphilic derivative T2
[0100] Image 6 It is the structural diagram of 3-((2-(dimethylamino)ethyl)(methyl)amino)propionic acid amphiphilic derivative T2; its preparation is specifically as follows: select N, N, N'-ethylenediamine, Oleic acid etc. are used as raw materials, synthesized according to the synthesis method of Example 1, the difference is: in step 1, the reactant 1 is oleic acid, and other synthesis steps, synthesis principles and raw materials are exactly the same. Obtain T2 lipid. All have been purified by HPLC and identified by mass spectrometry, the purity is greater than 95%, and the molecular weight is consistent with the theoretical value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com