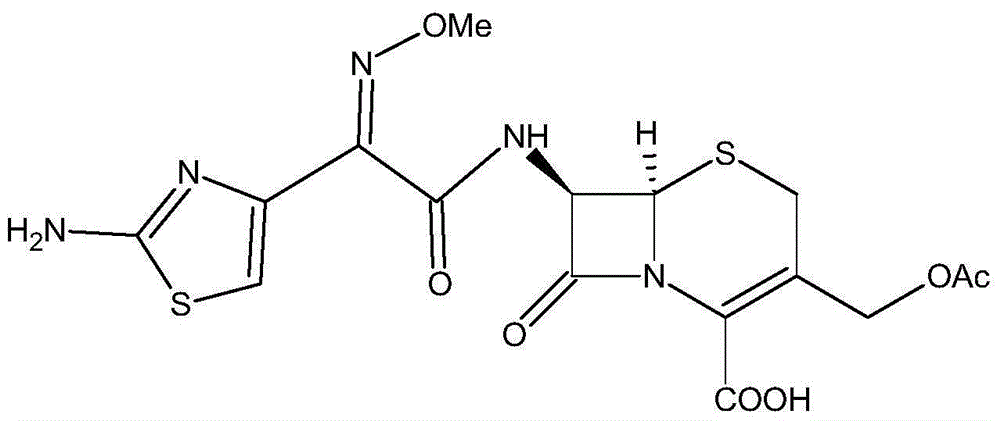
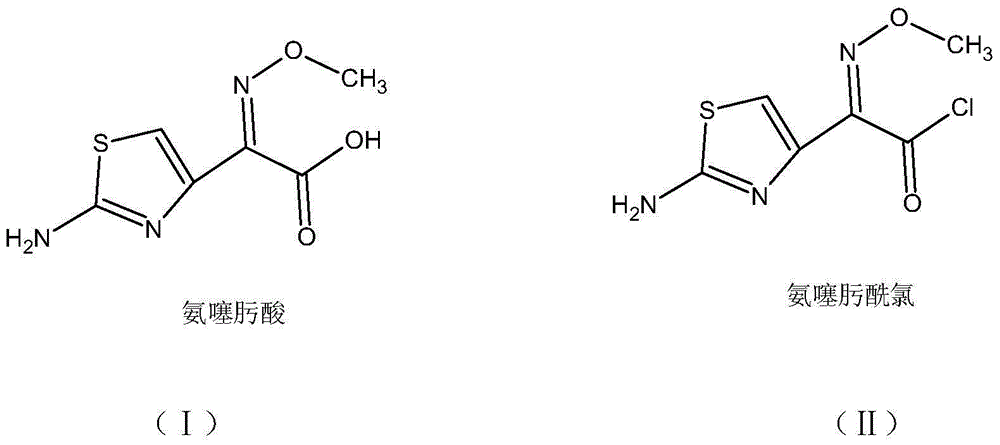
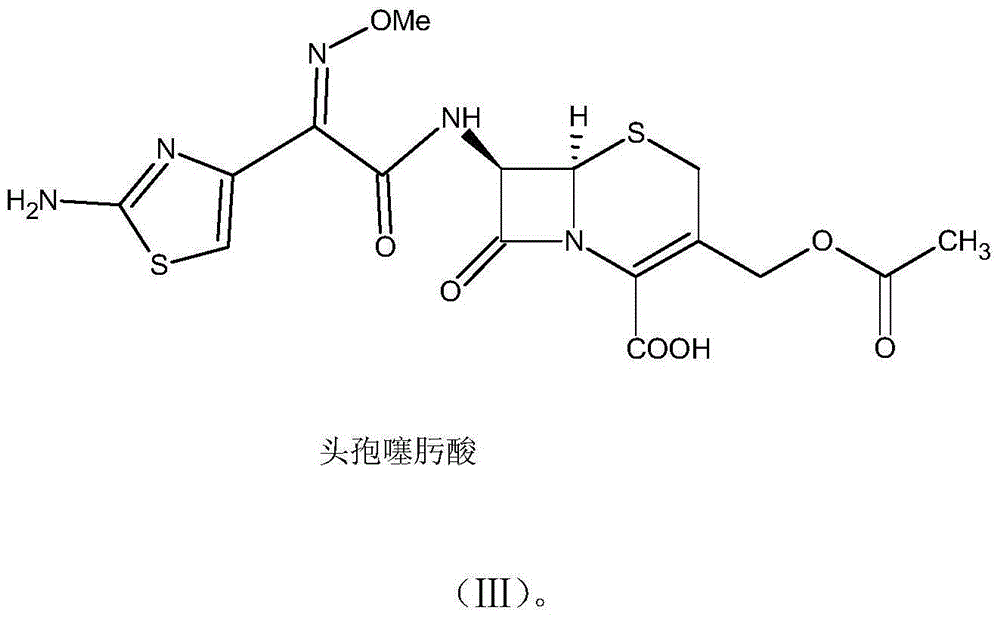
One-pot synthesis method of cefotaxime acid
A technology of cefotaxime acid and synthesis method, which is applied in the directions of organic chemistry, organic chemistry, etc., can solve problems such as potential safety hazards, difficulty in recovery, increase in production cost, etc., so as to ensure normal precipitation, reduce production cost and prolong service life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of one-pot synthesis method of cefotaxime acid, the steps are as follows:
[0039] (1) Add 25.0 g of aminothiaxamic acid into 250 ml of dichloromethane, cool down to -12°C, and keep warm to dissolve 11 ml of oxalyl chloride in 10.5 ml of N,N-dimethylformamide, add and keep warm for 2 hours to obtain Organic solution of oximyl chloride;
[0040] (2) Add 33.0g of 7-ACA and 10ml of water to the organic solution containing aminothioxamyl chloride, add 37ml of triethylamine dropwise at -5°C under temperature control, keep the reaction for 1.5h after dropping, add 400ml of water, stir to take the water phase, and use Formic acid was used to adjust the pH of the water phase to 2.0, and a solid was precipitated. The crystal was grown for 1 hour, and the temperature was lowered to 0° C. to grow the crystal. After conventional filtration, washing, and drying, 52.32 g of cefotaxime acid was obtained.
Embodiment 2
[0042] A kind of one-pot synthesis method of cefotaxime acid, the steps are as follows:
[0043] (1) Add 25.0 g of aminothioxamic acid into 250 ml of dichloromethane, cool down to -8°C, and keep warm; Organic solution of aminothioxamyl chloride;
[0044] (2) Add 33.0g of 7-ACA and 10ml of water to the organic solution containing aminothioxamyl chloride, add 37.1ml of triethylamine dropwise at -3°C under temperature control, keep the reaction for 2.5 hours after dropping, add 400ml of water, stir to take the water phase, Use acetic acid to adjust the pH of the aqueous phase to 2.5, precipitate solids, grow crystals for 1 hour, cool down to 2°C to grow crystals, perform conventional filtration, wash, and dry to obtain 51.38 g of cefotaxime acid.
Embodiment 3
[0046] A kind of one-pot synthesis method of cefotaxime acid, the steps are as follows:
[0047] (1) Add 25.0 g of aminothioxamic acid into 200 ml of chloroform, lower the temperature to 1 °C, add 11 ml of oxalyl chloride dissolved in 10.5 ml of N,N-dimethylformamide under temperature control, and keep warm for 2 hours to obtain Organic solutions of acid chlorides;
[0048] (2) Add 33.0g of 7-ACA and 10ml of water to the organic solution containing aminothioxamyl chloride, add 27.5ml of diethylamine dropwise at -1°C at temperature control, keep the reaction for 2 hours after dropping, add 350ml of water, stir to take the water phase, and use Acetic acid was used to adjust the pH of the water phase to 2.7, and a solid was precipitated. The crystal was grown for 1 hour, and the temperature was lowered to 3°C to grow the crystal. After conventional filtration, washing, and drying, 51.95 g of cefotaxime acid was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com