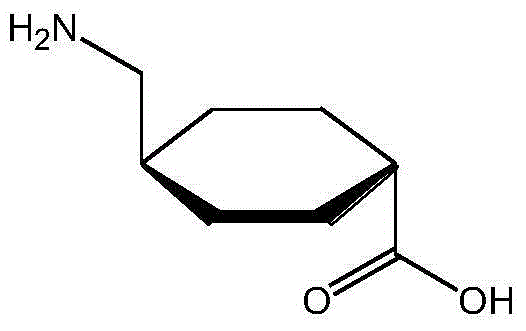
Technical formula and preparation method of tranexamic acid freeze-dried powder injection
A freeze-dried powder injection and tranexamic acid technology, applied in the chemical field, can solve the problems of unqualified tranexamic acid product quality and potential safety hazards, and achieve the effects of uniform and stable quality, low production cost, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription
[0021]
[0022] Measure tert-butanol into a container, add water for injection, stir and mix evenly, cool the mixed solvent to 8°C and keep it warm, then add tranexamic acid, stir to mix the solution evenly, after the mixed solution is checked and passed, use The peristaltic pump is sent to the sterile room and filtered through a 0.22 μm microporous membrane until it is clear, filled in a controlled antibiotic glass bottle, partially plugged with a butyl rubber stopper, and placed in a plate; the plated sample to be freeze-dried is placed in a freeze-dried In the box, the temperature of the sample is lowered to -35°C and kept for 3h, the vacuum system is turned on, and when the vacuum of the front box reaches below 20Pa, the freeze-drying is started, and the temperature of the shelf is raised to -10°C within 1 hour, and kept for 5h, then Raise the temperature of the shelf to 0°C within 1 hour and maintain it for 4h, raise the temperature of the shelf t...
Embodiment 2
[0024] prescription
[0025]
[0026] Measure tert-butanol into a container, add water for injection, stir and mix evenly, cool the mixed solvent to 8°C and keep it warm, then add tranexamic acid, stir to mix the solution evenly, after the mixed solution is checked and passed, use The peristaltic pump is sent to the sterile room and filtered through a 0.22 μm microporous membrane until it is clear, filled in a controlled antibiotic glass bottle, partially plugged with a butyl rubber stopper, and placed in a plate; the plated sample to be freeze-dried is placed in a freeze-dried In the box, the temperature of the sample is lowered to -35°C and kept for 3h, the vacuum system is turned on, and when the vacuum of the front box reaches below 20Pa, the freeze-drying is started, and the temperature of the shelf is raised to -10°C within 1 hour, and kept for 5h, then Raise the temperature of the shelf to 0°C within 1 hour and maintain it for 4h, raise the temperature of the shelf t...
Embodiment 3
[0028] prescription
[0029]
[0030] Measure tert-butanol into a container, add water for injection, stir and mix evenly, cool the mixed solvent to 8°C and keep it warm, then add tranexamic acid, stir to mix the solution evenly, after the mixed solution is checked and passed, use The peristaltic pump is sent to the sterile room and filtered through a 0.22 μm microporous membrane until it is clear, filled in a controlled antibiotic glass bottle, partially plugged with a butyl rubber stopper, and placed in a plate; the plated sample to be freeze-dried is placed in a freeze-dried In the box, the temperature of the sample is lowered to -35°C and kept for 3h, the vacuum system is turned on, and when the vacuum of the front box reaches below 20Pa, the freeze-drying is started, and the temperature of the shelf is raised to -10°C within 1 hour, and kept for 5h, then Raise the temperature of the shelf to 0°C within 1 hour and maintain it for 4h, raise the temperature of the shelf t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com