Fluorescent probe based on 8-aminoquinoline derivative and synthetic method and application thereof
A fluorescent probe, aminoquinoline technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of fluorescent probes that are rarely reported, application limitations, complex synthesis process, etc., and achieve product separation The purification process is easy, the synthesis method is simple, and the raw materials are easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Synthesize a reaction formula based on 8-aminoquinoline derivative fluorescent probe:
[0038]
[0039] (2) Synthesize a specific step based on 8-aminoquinoline derivative fluorescent probe:
[0040] Weigh 330mg of 2-chloro-N-(quinolin-8-yl)acetamide and 55mg of 2-propynylamine, dissolve in 20ml of acetonitrile, add 30mg of potassium iodide (KI) and 150mg of N,N-diisopropyl Diethylamine (DIPEA), refluxed for 10 hours, spin out acetonitrile, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, and the obtained oil was purified by silica gel column chromatography, and purified with ethyl acetate Separation with petroleum ether = 1:1 (v / v) as eluent to obtain 179 mg of light yellow solid compound, fluorescent probe L, with a yield of 75%.
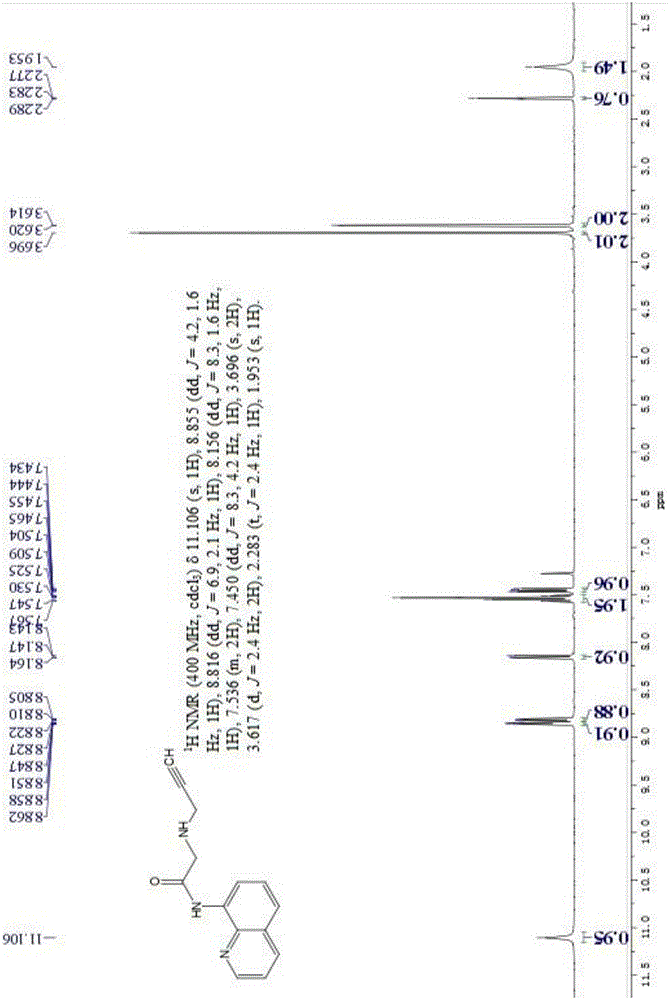
[0041] Basic data of fluorescent probe L:
[0042] 1 HNMR (400MHz, CDCl 3 )δ11.106(s,1H),8.855(dd,J=4.2,1.6Hz,1H),8.816(dd,J=6.9,2.1Hz,1H),8.156(dd,J=8.3,...
Embodiment 2
[0044] Weigh 396mg of 2-chloro-N-(quinolin-8-yl)acetamide and 82mg of 2-propynylamine, dissolve in 25ml of acetonitrile, add 35mg of potassium iodide (KI) and 165mg of N,N-diisopropyl diethylamine (DIPEA), refluxed for 15 hours, spin out acetonitrile, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, and the obtained oily matter was purified by silica gel column chromatography and purified with ethyl acetate Separation with petroleum ether = 1:3 (v / v) as eluent to obtain 280 mg light yellow solid compound, fluorescent probe L, with a yield of 78%.
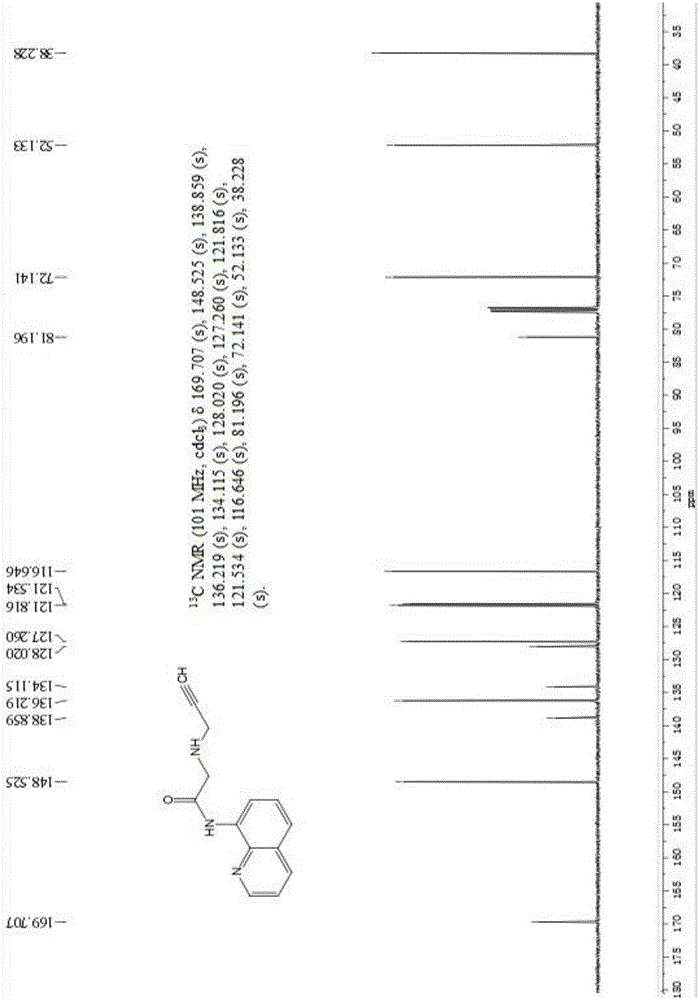
[0045] 13 CNMR (101MHz, CDCl 3 ( s), 81.196(s), 72.141(s), 52.133(s), 38.228(s), such as figure 2 .
Embodiment 3
[0047] Weigh 528mg of 2-chloro-N-(quinolin-8-yl)acetamide and 110mg of 2-propynylamine, dissolve in 35ml of acetonitrile, add 50mg of potassium iodide (KI) and 258mg of N,N-diisopropyl Diethylamine (DIPEA), refluxed for 18 hours, spin out acetonitrile, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, and the obtained oil was purified by silica gel column chromatography, and purified with ethyl acetate Separation with petroleum ether = 1:5 (v / v) as eluent to obtain 406 mg light yellow solid compound, namely fluorescent probe L, with a yield of 85%.
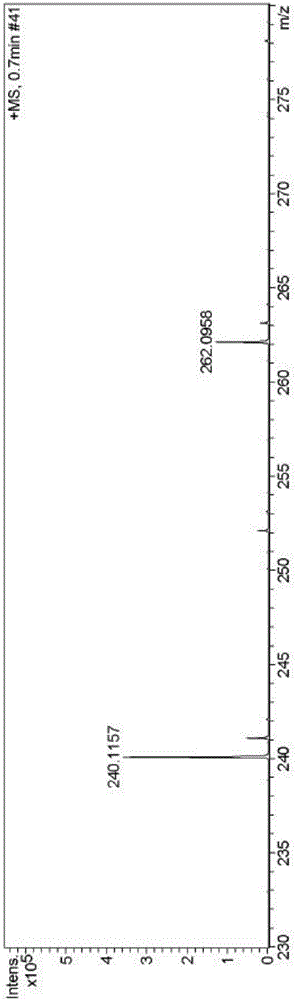
[0048] High resolution mass spectrum (electrospray, positive mode) of embodiment 3: calculated value of fluorescent probe L [L+H] + :240.1059, the measured value is 240.1157, such as image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com