A kind of aztreonam compound and preparation thereof
A technology of aztreonam and its compounds, applied in the field of aztreonam compounds and their preparations, can solve problems such as low industrialization efficiency, unsuitability for industrialization, and affecting product purity, and achieve increased solubility and dissolution speed, good stability, and flow sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
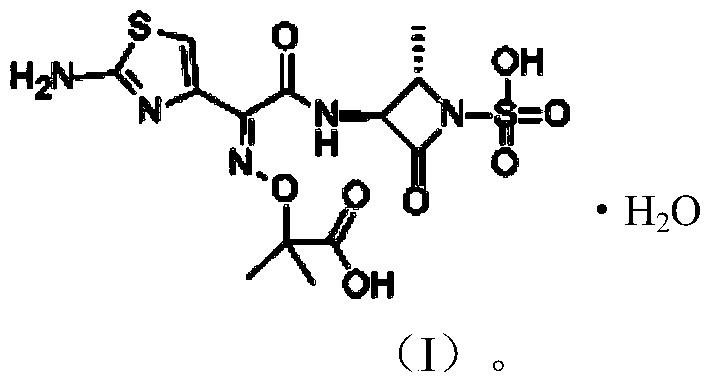
[0051] The preparation of embodiment 1 aztreonam monohydrate
[0052] 1. Grind the α crystal form of aztreonam, pass through a 300-mesh sieve, add it into 95% methanol solution, and heat up to 40-45°C while stirring; the weight ratio of aztreonam crude product to methanol solution is 1:30 ; Stirring speed is 240 rev / min; Add activated carbon, stir for 10 minutes and filter aseptically;
[0053] 2. Add ethyl acetate while stirring, and lower the temperature to 0°C at the same time; the stirring speed is 120 rpm; the weight of ethyl acetate is twice the weight of the methanol solution of aztreonam, and the adding speed is 80 ml / min; the cooling speed 1~2℃ / hour;
[0054] 3. After adding the mixed solvent, the obtained crystals were left to stand for crystallization; filtered, washed, and vacuum-dried for 8 hours to obtain aztreonam monohydrate.
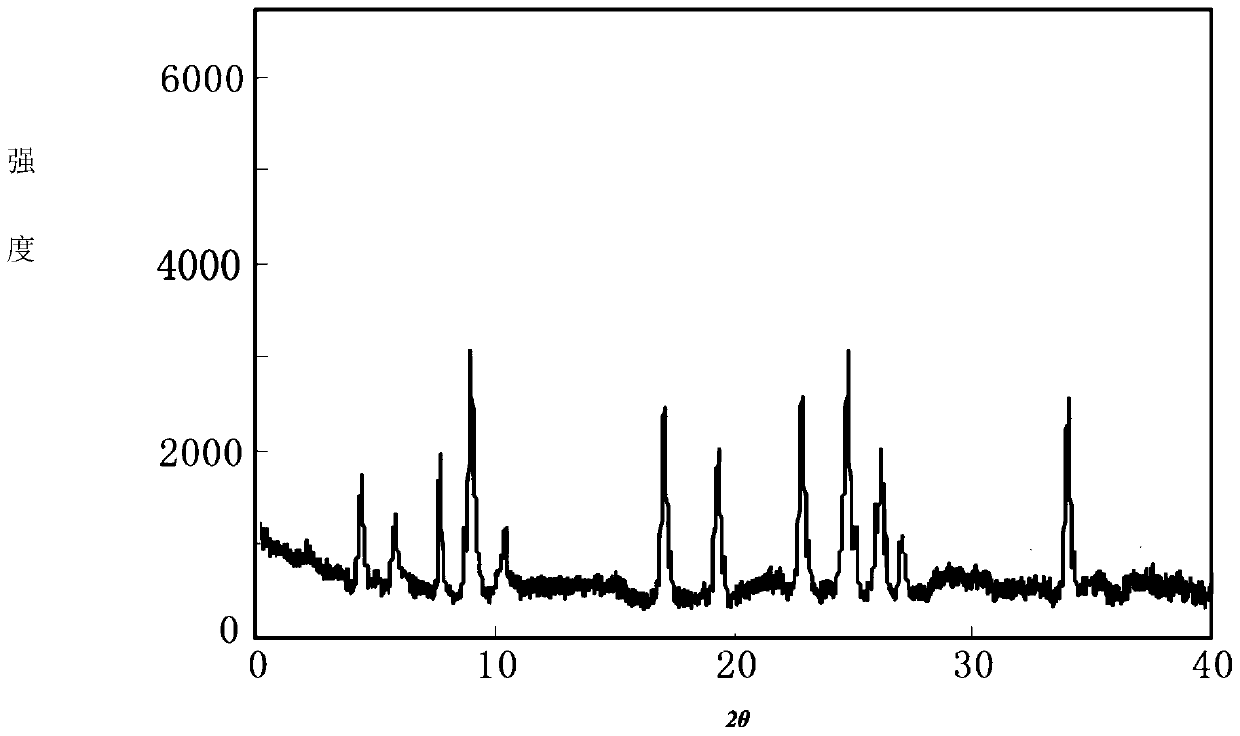
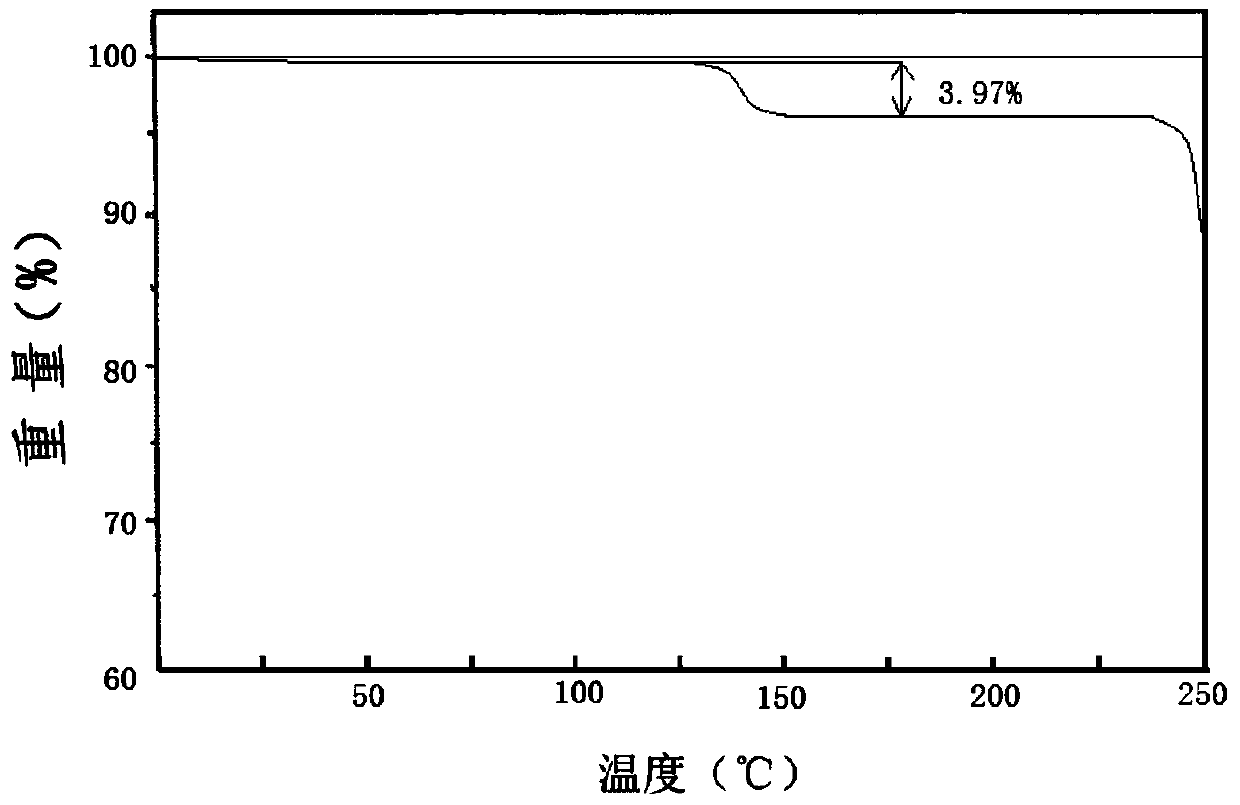
[0055] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.95%, and the yield is 90.6%; ...
Embodiment 2
[0056] The preparation of embodiment 2 aztreonam monohydrate
[0057] 1. Grind the β crystal form of aztreonam, pass through a 300-mesh sieve, add to 90% methanol solution, and heat up to 45°C while stirring; the weight ratio of aztreonam crude product to methanol solution is 1:25; Stirring speed is 360 rpm; add activated carbon, stir for 120 minutes and then sterile filter;
[0058] 2. Add ethyl acetate while stirring, and lower the temperature to -2°C at the same time; the stirring speed is 180 rpm; the weight of ethyl acetate is 3 times the weight of the methanol solution of aztreonam, and the adding speed is 70 ml / min; cool down The speed is 2°C / hour;
[0059] 3. After adding the mixed solvent, the obtained crystals were left to stand for crystallization; filtered, washed, and vacuum-dried for 6 hours to obtain aztreonam monohydrate.
[0060] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.90%, and the yield is 92.6%; the ...
Embodiment 3
[0061] The preparation of embodiment 3 aztreonam monohydrate
[0062] 1. Grind the α crystal form of aztreonam, pass through a 200-mesh sieve, add to 93% methanol solution, and heat up to 43°C while stirring; the weight ratio of aztreonam crude product to methanol solution is 1:25; Stirring speed is 360 rpm; add activated carbon, stir for 15 minutes and then sterile filter;
[0063] 2. Add ethyl acetate while stirring, and lower the temperature to 2°C at the same time; the stirring speed is 180 rpm; the weight of ethyl acetate is 3 times the weight of the methanol solution of aztreonam, and the adding speed is 70 ml / min; the cooling speed 1.5°C / hour;
[0064] 3. After adding the mixed solvent, the obtained crystals were left to stand for crystallization; filtered, washed, and vacuum-dried for 7 hours to obtain aztreonam monohydrate.
[0065] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.91%, and the yield is 90.3%; the X-ray...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com