Synthetic method of 2-bromo-5-iodotoluene
A synthesis method and technology of iodotoluene, which is applied in the field of synthesis of 2-bromo-5-iodotoluene, can solve the problems of unsuitability for large-scale production and low efficiency, and achieve reliable oxidation performance, improved yield and quality, and less difficult control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
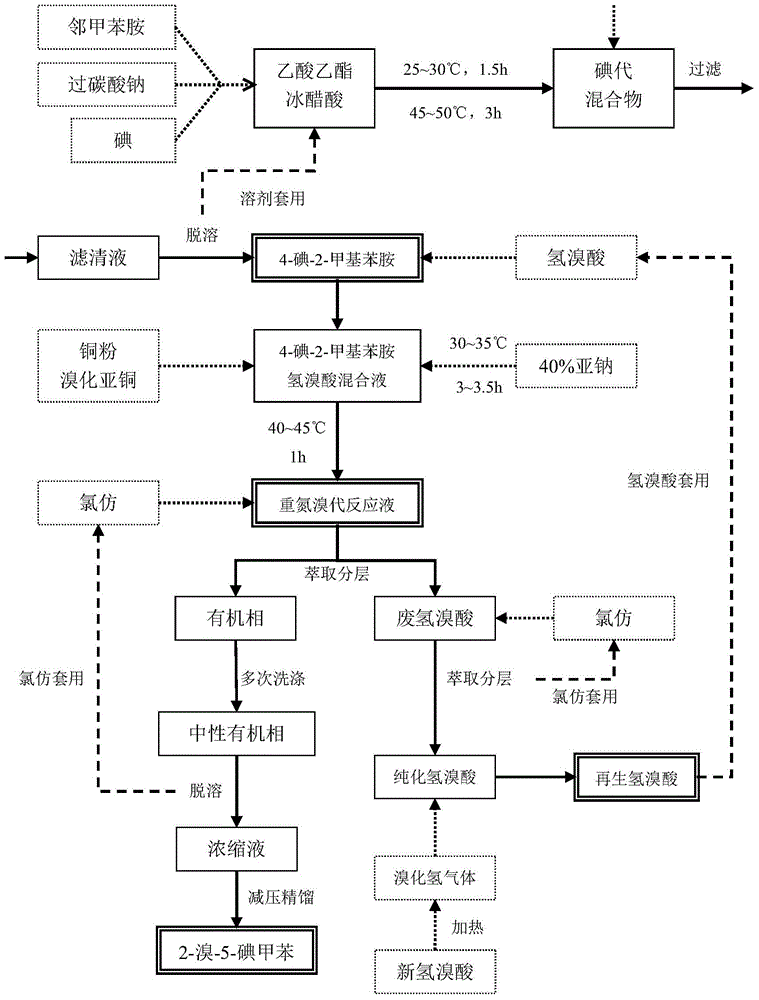
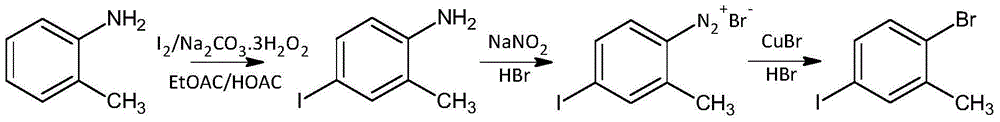
[0046] Step 1 Iodo
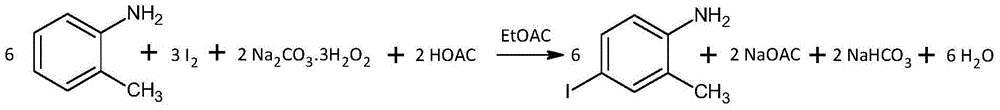
[0047]Put 82mL of ethyl acetate and 101mL of glacial acetic acid into a 500mL four-neck flask, then put in 20.43g of iodine powder, 13.57g of sodium percarbonate, and 18.41g of o-toluidine. There is an exotherm from acid-base neutralization, and stir under normal water cooling conditions. , to obtain a dark brown clear solution with a small amount of small solid particles of sodium acetate suspended. Use a constant temperature water bath to keep the reaction temperature at 27°C±1°C for 1 hour and 30 minutes, then heat the water bath to 53°C to raise the temperature of the reaction solution to 48°C, and continue to keep warm for 2 hours and 32 minutes. After 2 hours and 32 minutes, the color of the reaction solution has changed significantly. shallow. Take the reaction liquid onto the filter paper and observe for 5 minutes. The brown spots are stable in color and there is no fading caused by iodine sublimation, indicating that the reaction of iodine is bas...
Embodiment 2
[0051] Step 1 Iodo
[0052] Put 323mL of ethyl acetate and 406mL of glacial acetic acid into a 2000mL four-neck flask, then put in 81.23g of iodine powder, 53.84g of sodium percarbonate, and 73.21g of o-toluidine, and stir under normal water cooling conditions to obtain a dark brown clear solution, suspend A small amount of sodium acetate solid small particles. Use a constant temperature water bath to keep the reaction temperature at 29°C±1°C for about 1 hour and 30 minutes, then heat the water bath to 54°C, and the temperature of the reaction solution rises to 49°C, and continue to keep warm for about 3 hours. Add 6.23g of sodium sulfite, stir for 30 minutes and then filter off the insoluble salt. Heat the filtrate with a water bath, distill off the mixed solvent under normal pressure, and then switch to vacuum distillation at -0.075Mpa. After about 40 minutes, almost no solvent is evaporated, and finally vacuum -0.085Mpa, water bath 96.7°C, bottle The internal gas phase te...
Embodiment 3
[0056] Step 1 Iodo
[0057] 407 mL of ethyl acetate and 502 mL of glacial acetic acid were put into a 2000 mL four-neck flask, and then 101.84 g of iodine powder, 67.08 g of sodium percarbonate, and 91.44 g of o-toluidine were added. Use a constant temperature water bath to keep the reaction temperature at 29°C±1°C for about 1 hour and 30 minutes, then heat the water bath to 54°C, and the temperature of the reaction solution to 48°C, and continue to keep warm for about 3 hours. Add 7.36g of sodium sulfite, stir for 30 minutes and filter off insoluble salts. Under heating in a water bath, evaporate the mixed solvent from the filtrate under normal pressure, and then switch to vacuum distillation at -0.075Mpa. After about 40 minutes, almost no solvent is evaporated, and finally vacuum -0.085Mpa, water bath 96.4°C, in the bottle The gas phase temperature is about 87°C, and the tan viscous liquid remaining in the bottle is 4-iodo-2-methylaniline.
[0058] Step 2 diazotization of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com