Application of anti-depression compound on preparation of anti-depression drugs and anti-depression health-care foods
A health food, antidepressant technology, applied in the field of medicine, can solve the problems of slow onset (it takes weeks or even months, resistance to treatment, slow onset (it takes weeks or even months to slow down the effect), etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
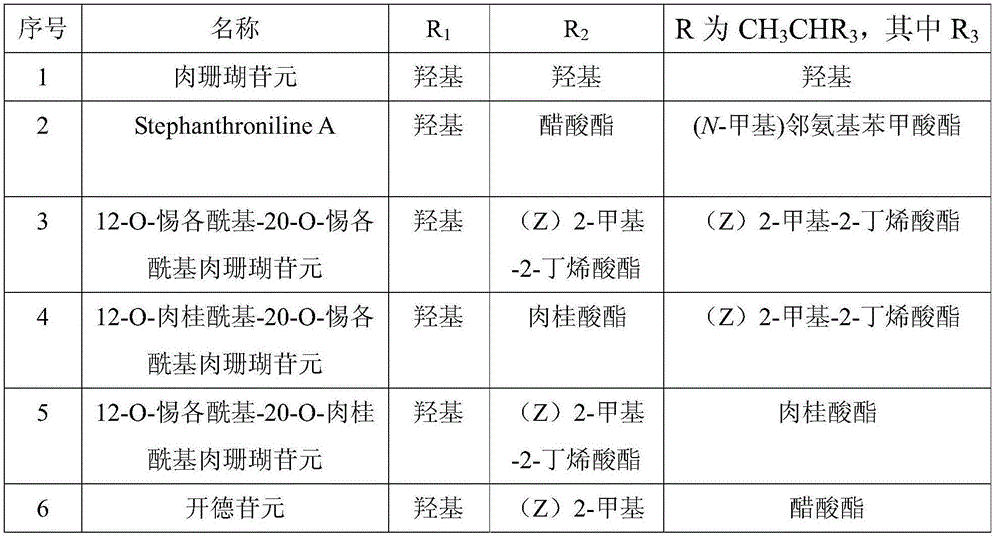
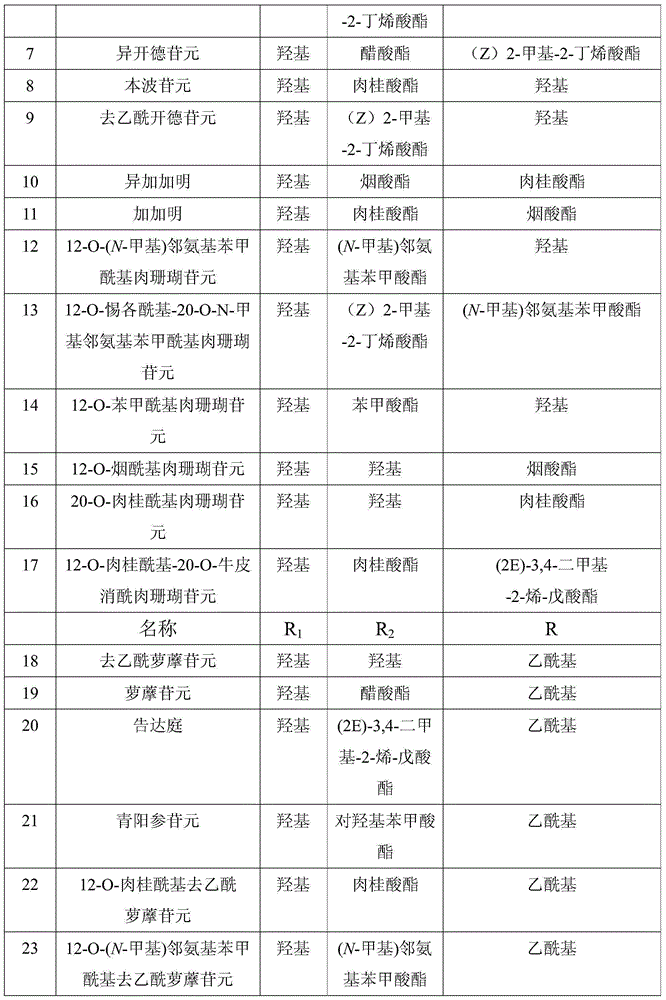
[0033] Example 1: Preparation and structure identification of sarcoclindin
[0034] Taishan Radix Polygoni Multiflori 5kg is pulverized, and ethanol aqueous solution (water-containing volume percentage is 5%) percolates, obtains ethanol extract, and ethanol extract is extracted with ethyl acetate, and ethyl acetate extract is dissolved in 0.2N sulfuric acid methanol solution (being sulfuric acid solution). Form a sulfuric acid methanol solution in methanol, wherein the concentration of sulfuric acid in the sulfuric acid methanol solution is 0.2mol / L) Hydrolyze at 70°C for 5 hours, neutralize with sodium bicarbonate, concentrate, extract the concentrate with ethyl acetate, the extract is the total aglycone. 180 g of total aglycones were subjected to silica gel column chromatography, and gradient elution was performed with a dichloromethane-methanol system (dichloromethane to methanol volume ratio 100:0→40:60) to obtain Fr1-Fr8. Fr8 15g was subjected to repeated column chromatog...
Embodiment 2
[0036] Example 2: Preparation and structure identification of deacetylradixin
[0037] Taishan Baishouwu 5kg is pulverized, percolated with ethanol aqueous solution (water content is 5%), the ethanol extract is extracted with ethyl acetate, and the ethyl acetate extract is hydrolyzed with 0.2N sulfuric acid methanol at 70°C for 5 hours, and dissolved in sodium bicarbonate And, concentrated, the concentrate was extracted with ethyl acetate, and the extract was the total aglycone. 180 g of total aglycones were subjected to silica gel column chromatography, and gradient elution was performed with a dichloromethane-methanol system (100:0→40:60) to obtain Fr1-Fr8. 15g of Fr8 was subjected to repeated column chromatography on Rp-18, eluted with methanol-water system (53%→60%), detected by TLC, the same fractions were combined, and recrystallized from methanol to obtain 675 mg of deacetylradixaglycone.
[0038] Deacetylradixaglycone, C 21 h 32 o 6 , Colorless needles (methanol). ...
Embodiment 3
[0039] Example 3: Preparation and structure identification of diglucarinin
[0040] Taishan Baishouwu 5kg is pulverized, percolated with ethanol aqueous solution (water content is 5%), the ethanol extract is extracted with ethyl acetate, and the ethyl acetate extract is hydrolyzed with 0.2N sulfuric acid methanol at 70°C for 5 hours, and dissolved in sodium bicarbonate And, concentrated, the concentrate was extracted with ethyl acetate, and the extract was the total aglycone. 180 g of total aglycones were subjected to silica gel column chromatography, and gradient elution was performed with a dichloromethane-methanol system (100:0→40:60) to obtain Fr1-Fr8. Rp-18 on 10 g of Fr6 was subjected to repeated column chromatography, eluted with methanol-water system (53%→60%), detected by TLC, and the same fractions were combined and recrystallized from methanol to obtain 1.6 g of diglucarinin.
[0041] Radixanthin, C 23 h 34 o 7 , Colorless needles (methanol). IR(KBr): 3510, 169...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com