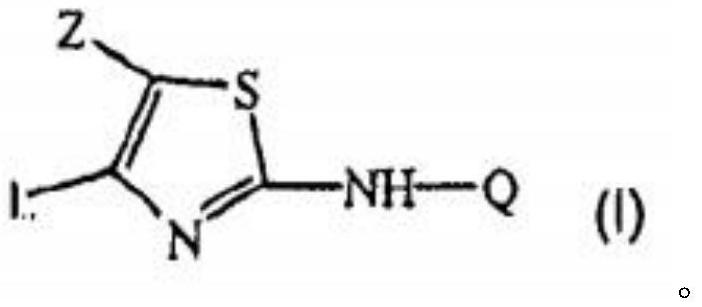
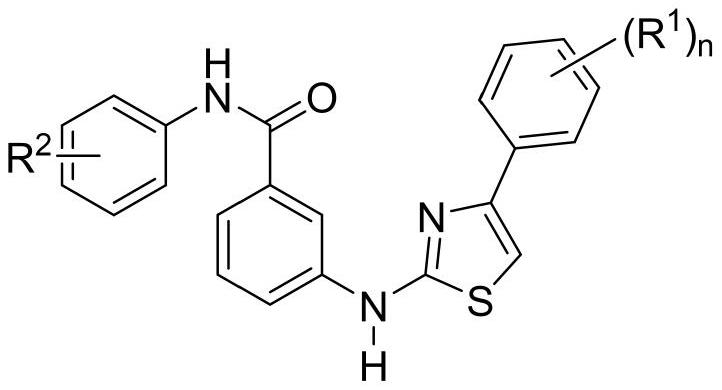
A kind of thiazolamide derivative and its antidepressant use
A technology for aniline and compounds, applied in the field of preparation of thiazolamine derivatives, to achieve the effects of mild conditions, good antidepressant activity, and simple preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 3-[(5-ethyl-4-phenylthiazol-2-yl)amino]benzoic acid
[0039] Step 1: 3-[(-4-phenylthiazol-2-yl)amino]benzoic acid
[0040]
[0041] Add 1.96g (0.01mol) 3-carboxyphenylthiourea, 1.99g (0.01mol) 2-bromo-1-phenylethanone and 15mL glacial acetic acid to a 100mL oblique reaction flask with a condenser, stir evenly, Heating to reflux, TLC (developing solvent: ethyl acetate: petroleum ether=4:1) monitoring the reaction progress, the reaction was about 24h. Remove the insoluble solids in the reaction flask while it is still hot, and rotate part of the solvent. It was put into a fume hood to cool at room temperature, the solid was precipitated, filtered, the obtained solid was dried, and 2.02 g of powder was weighed, and the yield was 68.2%. 1 H NMR (DMSO-D 6 , 400MHz), δ: 7.16 (s, 1H, CH-S), 7.30-8.24 (m, 9H, C 6 H 4 , C 6 H 5 ), 10.38 (s, 1H, COOH).
[0042] Step 2: Preparation of the compound of formula I
[0043]
[0044] Add (0.28g, 3mmol) aniline, ...
Embodiment 2
[0046] Example 2 Antidepressant activity test
[0047]In this experiment, the high-throughput selection method of fluorescent substance ASP was used to find compounds that directly act on or produce reuptake inhibitory effects through adenosine receptors. Fluorescent dye ASP+ is a neurotoxic compound that can be combined with monoamine transporters to enter cells and emit yellow fluorescence (the order of binding is dopamine transporter>adrenaline transporter>5-HT transporter). When there are other compounds that can bind to the transporter, it competes with ASP, thereby reducing the number of cells entering the cell, and the yellow fluorescence is weakened.
[0048] RBL cells are a cell line that can secrete histamine and 5-HT, and the cells are immortalized mast cells that can release the transmitters quantumly. And has the ability to re-uptake 5-HT.
[0049] CACO-2 cells are adenocarcinoma cell lines derived from human small intestine. Studies have shown that the cells ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com