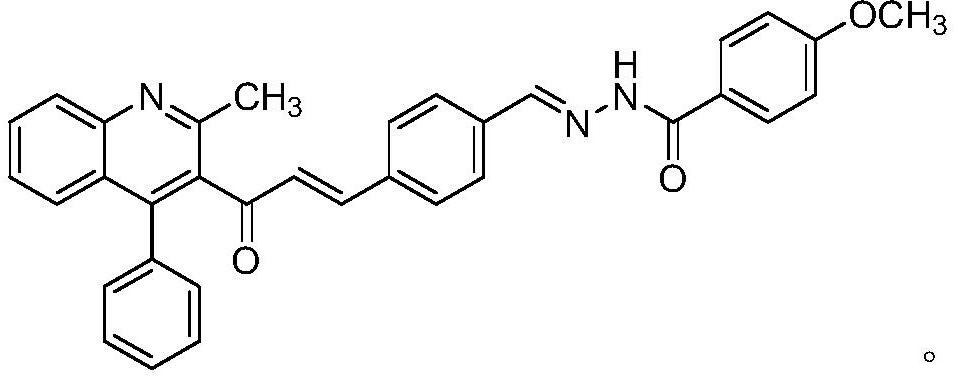
Anti-depression active substance BHC-one as well as preparation and application thereof
A drug and compound technology, applied in the compound field, can solve problems such as side effects, low drug reaction rate, and long onset time, and achieve good antidepressant activity, less impurity content, and less harmful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
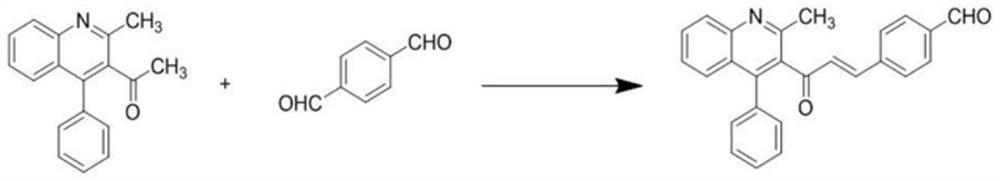
[0027] Step 1: Synthesis of 2-methyl-4-phenylquinoline-3-ethanone
[0028] Weigh 0.5g (5mmol) of acetylacetone and 0.48g (2.5mmol) of citric acid in a round bottom flask, and weigh 1.0g (5mmol) of 2-aminobenzophenone after dissolving it with 1,4-dioxane , slowly dripped into the round bottom flask while stirring on the magnetic stirrer, heated to 101 °C, judged the reaction process by thin layer chromatography (TLC), and reacted for about 12 hours. After the reaction was completed, pour the reaction solution into the beaker and cool After reaching room temperature, the pH was adjusted to weak alkalinity with 1M NaOH solution, and solids were precipitated. Suction filtration, washing with 0.1M dilute hydrochloric acid solution, washing with water, and drying the filter cake to obtain 2-methyl-4-phenylquinoline-3-ethanone;
[0029] Step 2: Synthesis of (E)-4-(3-(2-methyl-4-phenylquinolin-3-yl)-3-oxoprop-1-en-1-yl)benzaldehyde
[0030] Weigh 0.2g of terephthalaldehyde in a 100m...
Embodiment 2
[0038]All the experimental animals are ICR white mice, both male and female, with a body weight of 20g±2g. Before the experiment, they were stably raised in the SPF grade animal room for one week to adapt to the new feeding environment. The ventilation facilities in the animal room are running well, and the air supply and exhaust equipment are operating normally; the humidity is suitable (45-65%); the temperature is controlled at about 24°C; the noise is controlled within 60dB. The light conditions in the animal room were good, and the cycle of light and dark was controlled to be 12h per cycle. Move to the pharmacology laboratory within 1-2 hours before the experiment to adapt to the experimental conditions.
[0039] 2.1 Forced swimming experiment
[0040] Male mice of 20g±2g were taken and randomly divided into groups of 8 mice. The mice in the first group were injected with 100 mg / kg of compound BHC-one, the mice in the second group were injected with 100 mg / kg of fluoxet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com