Method for determining content of nerve growth factor (NGF) in nerve growth factor preparation
A technology of nerve growth factor and determination method, which is applied in the field of determining the content of nerve growth factor in nerve growth factor preparations by gel chromatography, can solve the problems of difficult separation of NGF and albumin, inability to accurately quantify, etc., and achieve the reduction of solvent usage Small size, shortened detection time, and high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Determination of NGF content in the preparation containing human serum albumin NGF by external standard method
[0054] 1. Chromatographic conditions:
[0055] Instrument: Waters ACQUITY H-Class Bio ultra-high performance liquid chromatography;
[0056] Gel chromatography column: Waters UPLC SEC BEH125, 125 Angstrom (1.7μm), 4.6×150mm;
[0057] Detector: UV detector, the detector uses a titanium flow cell;
[0058] Mobile phase: acetonitrile: 0.25M phosphate buffer solution = 15:85, pH value: 7.0, flow rate: 0.2ml / min; column temperature: 40°C; wavelength: 214nm; injection volume: 2μl.
[0059] 2. Experimental steps:
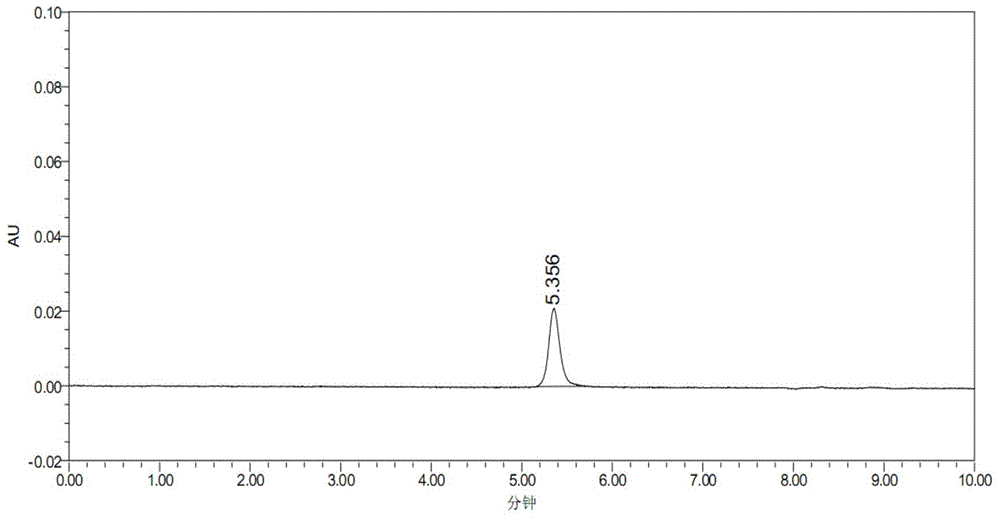
[0060] 1. Determination of control spectrum
[0061] Get the NGF stock solution of known concentration, add mobile phase and be mixed with the solution of 50 μ g / ml, as NGF contrast solution; Get mobile phase and corresponding adjuvant solution (containing 0.1 volume % human serum albumin, 5.0 volume % % mannitol) as a blank control, ea...
Embodiment 2
[0073] Embodiment 2: Dealbumin-depleted nerve growth factor NGF content is determined by external standard method
[0074] 1. Chromatographic conditions:
[0075] With embodiment 1.
[0076] 2. Experimental steps:
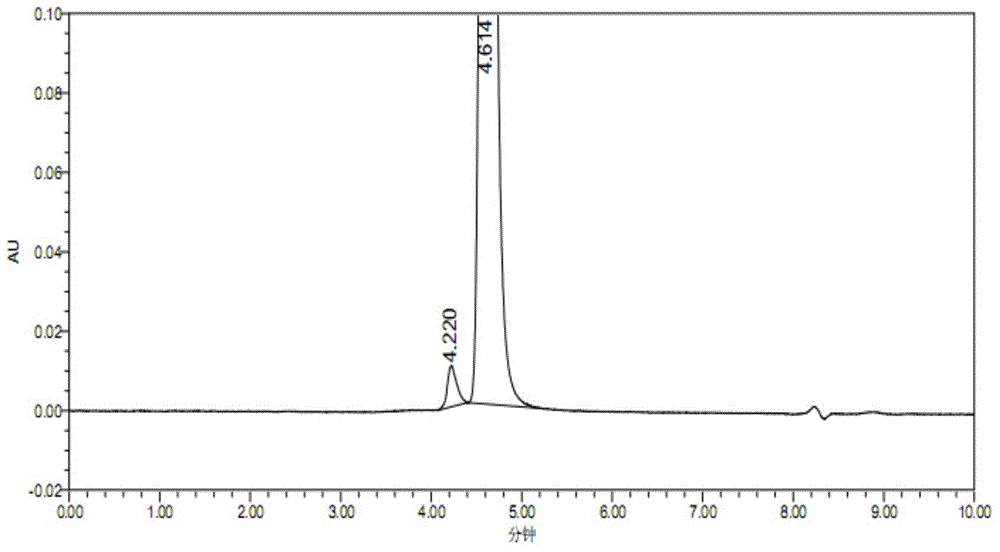
[0077] 1. Determination of control spectrum
[0078] The experimental procedure is the same as in Example 1. Take 2 μl of the corresponding adjuvant solution (containing 10 mg / ml amino acid, 5 volume % mannitol, and 50 mM disodium hydrogen phosphate) in the NGF preparation without albumin as a blank control, and detect it by the above method to determine the adjuvant The chromatographic peak position of each component in . The result is as Figure 5 As shown, the peak retention time of the excipients of the preparation far exceeds the retention time of the NGF contrast, and the interference of the excipients can also be excluded.
[0079] 2. Dealbumin NGF preparation
[0080] Take the NGF stock solution with known concentration, add mobile phase to prepare a ...
Embodiment 3
[0090] Embodiment 3: the recovery test of NGF content in the preparation by external standard method
[0091] 1. Chromatographic conditions:
[0092] With embodiment 1.
[0093] 2. Experimental steps:
[0094] 1, the preparation of contrast solution: get the known NGF stoste of concentration (specific source is the same as embodiment 1), add mobile phase and be mixed with the solution of 50 μ g / ml, as NGF contrast solution, prepare 2 parts, shake up, stand-by.
[0095] 2, the preparation of sample solution: get the auxiliary material solution containing human albumin (0.1 volume %) and mannitol (5.0 volume %) and each 10ml of the auxiliary material solution containing amino acid (10mg / ml) and mannitol (5 volume %) and an appropriate amount of NGF stock solution to prepare NGF solutions containing human serum albumin and de-albumin, respectively, wherein the concentrations of NGF were 40, 50, and 60 μg / ml, respectively, and 3 parts were prepared for each concentration, shaken...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com