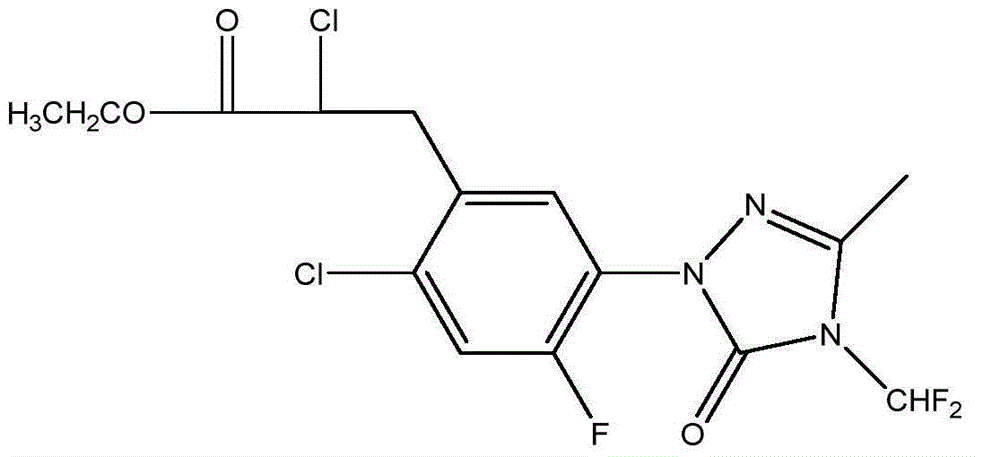
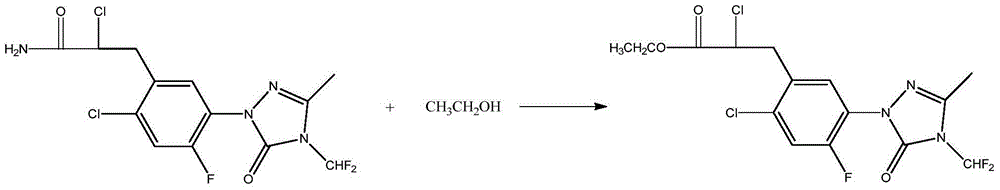Preparation method of carfentrazone-ethyl
A technology of mefentrazone and difluoromethyl, which is applied in the field of compound preparation, can solve the problems of difficult refining methods to obtain high-content products, poor production environment, and many impurities, and achieve low odor and low cost of raw materials and production environment , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazole- Preparation of 1-yl]-4-fluorophenyl}propanamide
[0044] Drop into 1-(5-amino-4-chloro-2-fluorophenyl)-4-difluoromethyl-3-methyl-1H-1,2,4,-triazole-5-one ( For the preparation method, see US5256793) 58.5 grams, 250 grams of acetone, stirring, controlled at 5 °C, 18 grams of hydrogen chloride, and sequentially added 28.4 grams of acrylamide, 3 grams of cuprous chloride, 4.8 grams of potassium chloride, cooled to -5 °C , control the temperature at -5°C to 0°C and add 20.7 grams of 40% sodium nitrite aqueous solution dropwise, and continue stirring for 1 hour after the addition is completed. After the reaction, add 100ml of toluene and 150ml of water, and stand at 20°C to separate the organic layer. Cool down to -10°C, crystallize and filter to obtain 58.1 g of solid. The crude product was recrystallized with 60 grams of ethanol, filtered, and dried to obtain 49.8 grams of 2-chloro-3...
Embodiment 2
[0046] 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazole- Preparation of 1-yl]-4-fluorophenyl}propanamide
[0047] Put 1-(5-amino-4-chloro-2-fluorophenyl)-4-difluoromethyl-3-methyl-1H-1,2,4,-triazol-5-one 58.5 into the reaction bottle gram, 250 grams of acetone and stirring, followed by adding 28.4 grams of acrylamide, 3 grams of cuprous chloride, and 4.8 grams of potassium chloride, cooling to -5°C, controlling the temperature at -5°C to 0°C, and adding 40% nitrous acid dropwise at the same time 20.7 grams of sodium aqueous solution and 48.7 grams of 30% hydrochloric acid solution, after the dropwise addition, continue to stir and react for 1 hour. After the reaction, add 100ml of toluene and 150ml of water, stand at 20°C to separate the organic layer, cool to -10°C, and crystallize and filter 54.1 g of solid were obtained. The crude product was recrystallized with 55 grams of ethanol, filtered, and dried to obtain 45.0 grams of 2-chlor...
Embodiment 3
[0049] Preparation of Metofen
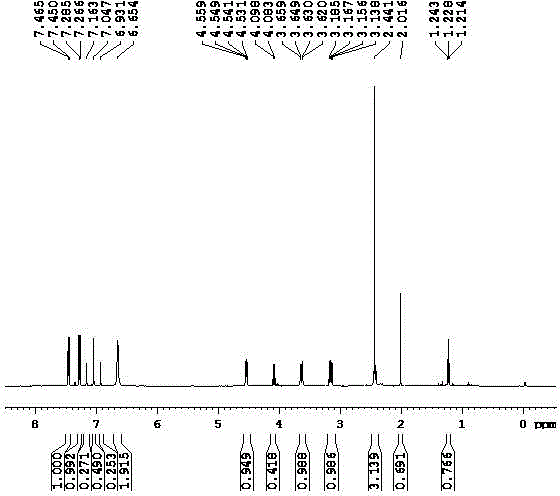
[0050] 2-chloro-3-{2-chloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H obtained by dropping into 76.5 grams of example 1 in the reaction bottle -1,2,4-triazol-1-yl]-4-fluorophenyl}propionamide, 240 grams of ethanol, 20 grams of concentrated sulfuric acid, reflux reaction for 24 hours, and a small amount of hydrochloric acid gas, stop until white turbidity appears Pass through, continue to react for 2 hours, distill out ethanol, lower the temperature, wash the light yellow liquid with 5% potassium carbonate aqueous solution and water respectively, and obtain 80.1 grams of light yellow liquid after drying, the quantitative content is more than 97.4%, and the yield is 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com