Clean Production Method of Methionine
A production method and technology of methionine, which is applied in the field of clean production technology of methionine, can solve the problems such as the difficulty of separating methionine analog salts, achieve the effects of high purity and yield, clean and environmentally friendly production, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
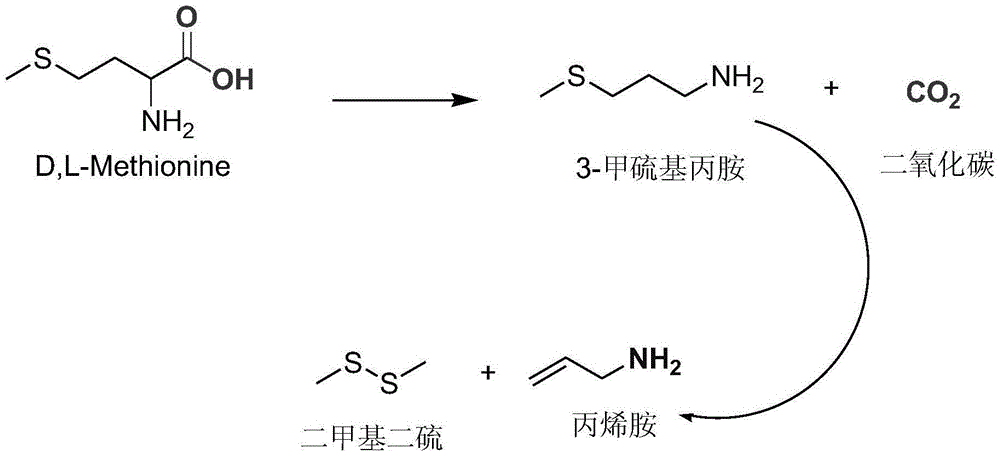
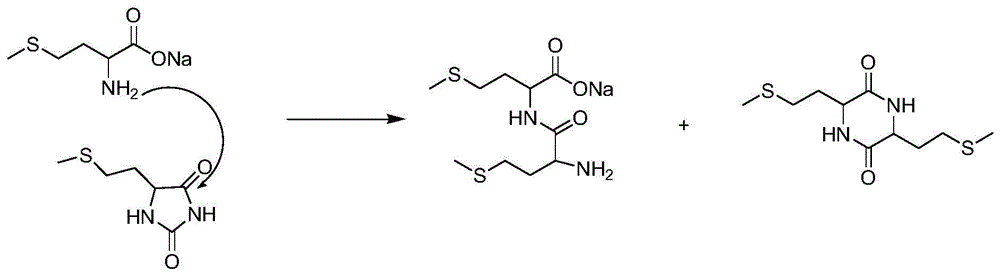
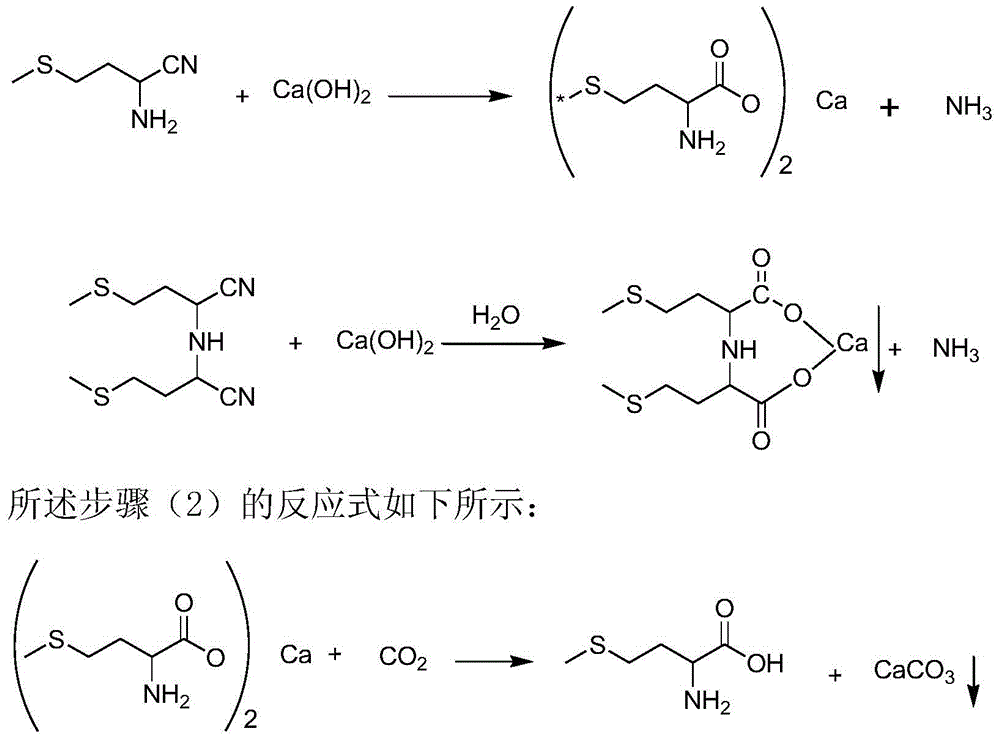
[0039] At 40°C, introduce 113.3g of 60% ammonia water (4mol) into a 500ml autoclave, then add 1mol of 2-hydroxy-4-methylthiobutyronitrile with a pump within 2 minutes, stirring The temperature was raised to 60° C., and the pressure of the reaction system reached 1.5 MPa. Stirring was continued at this temperature for 40 minutes, then the temperature was immediately cooled to room temperature, and the pressure was reduced to normal pressure. The mixture in the reaction system was analyzed by HPLC, showing that 99.2% of 2-amino-4-methylthiobutyronitrile was formed, and 0.8% of 2,2'-bis-(2-methylthioethyl)iminodiacetonitrile was formed.
[0040] The 2-amino-4-methylthiobutyronitrile aqueous solution obtained above is directly added dropwise in the emulsion of calcium hydroxide (1.5mol), the mass ratio of calcium hydroxide and water in this emulsion is 1:4, and the reaction temperature is 80°C, controlled dropwise for about 2 hours, while stirring to discharge ammonia; fully hydr...
Embodiment 2
[0043] At 40°C, introduce 113.3g of 60% ammonia water (4mol) into a 500ml autoclave, then add 1mol of 2-hydroxy-4-methylthiobutyronitrile with a pump within 2 minutes, stirring The temperature was raised to 60° C., and the pressure of the reaction system reached 1.5 MPa. Stirring was continued at this temperature for 40 minutes, then the temperature was immediately cooled to room temperature, and the pressure was reduced to normal pressure. The mixture in the reaction system was analyzed by HPLC, showing that 99.2% of 2-amino-4-methylthiobutyronitrile was formed, and 0.8% of 2,2'-bis-(2-methylthioethyl)iminodiacetonitrile was formed.
[0044] The 2-amino-4-methylthiobutyronitrile aqueous solution obtained above is directly added dropwise in the emulsion of calcium hydroxide (1.5mol), the mass ratio of calcium hydroxide and water in this emulsion is 1:4, and the reaction temperature is 80°C, controlled dropwise for about 2 hours, while stirring to discharge ammonia; fully hydr...
Embodiment 3
[0048] At 45°C, introduce 85g of 80% ammonia water (4mol) into a 250ml autoclave, then add 1mol of 2-hydroxy-4-methylthiobutyronitrile with a pump within 2 minutes, and heat up while stirring At 60°C, the pressure of the reaction system reaches 2.0 MPa. Stirring was continued at this temperature for 40 minutes, then the temperature was immediately cooled to room temperature, and the pressure was reduced to normal pressure. The mixture in the reaction system was analyzed by HPLC, showing that 99.5% of 2-amino-4-methylthiobutyronitrile was formed, and 0.5% of 2,2'-bis-(2-methylthioethyl)iminodiacetonitrile was formed.
[0049] The 2-amino-4-methylthiobutyronitrile aqueous solution obtained above is directly added in the 1L autoclave that calcium hydroxide (1.5mol) emulsion is housed, and the mass ratio of calcium hydroxide and water in this emulsion is 1: 4. The reaction temperature is 100°C, heat preservation and stirring for about 2 hours, fully hydrolyzed, then release the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com