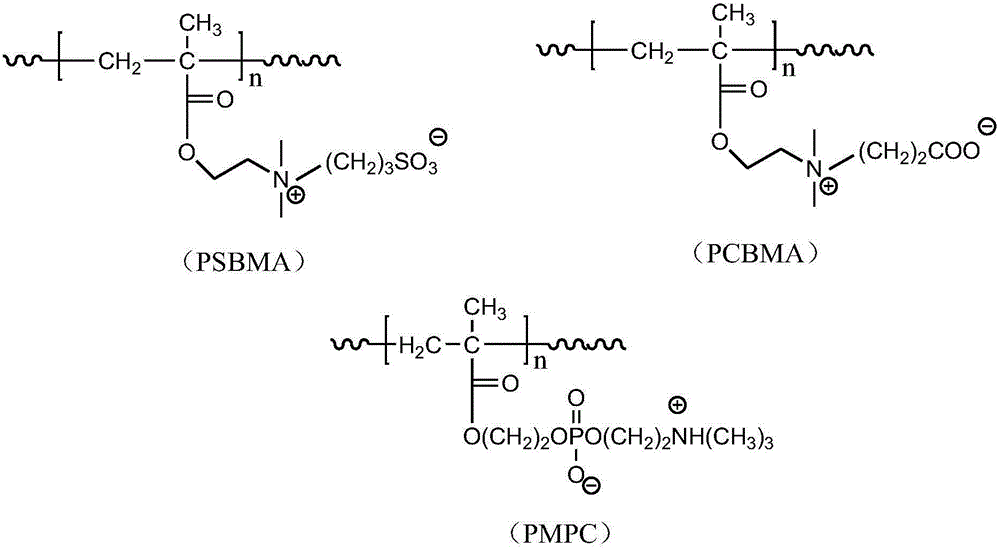
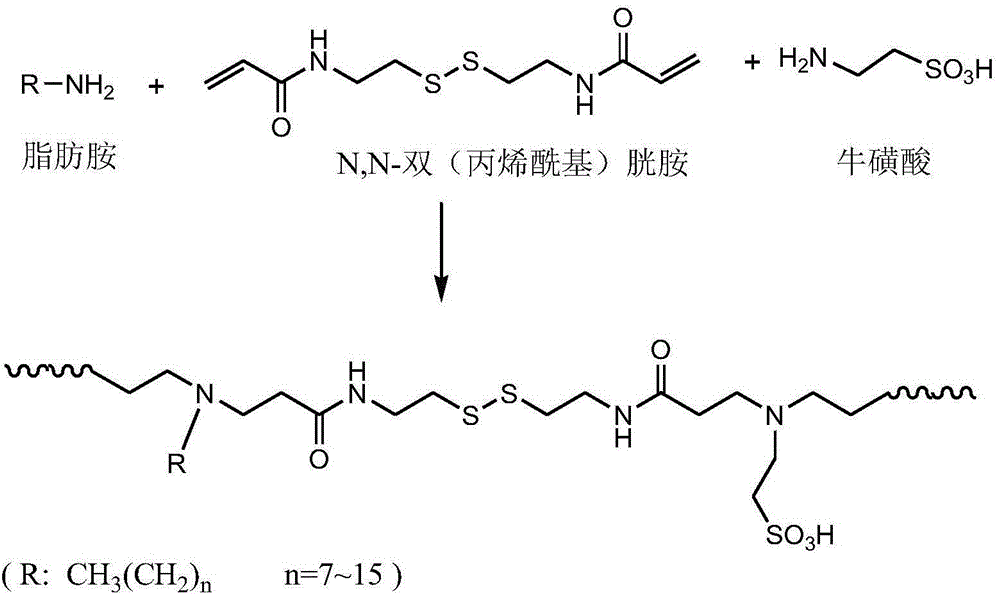
Reductively degradable polyzwitterionic nano-micelle and preparation method thereof
A polyzwitterion and nanomicelle technology, which is applied in the directions of pharmaceutical formulations, inactive medical preparations, antitumor drugs, etc., can solve problems such as unsatisfactory use and difficulty in excretion, and achieve complete reaction, simple method and product. pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
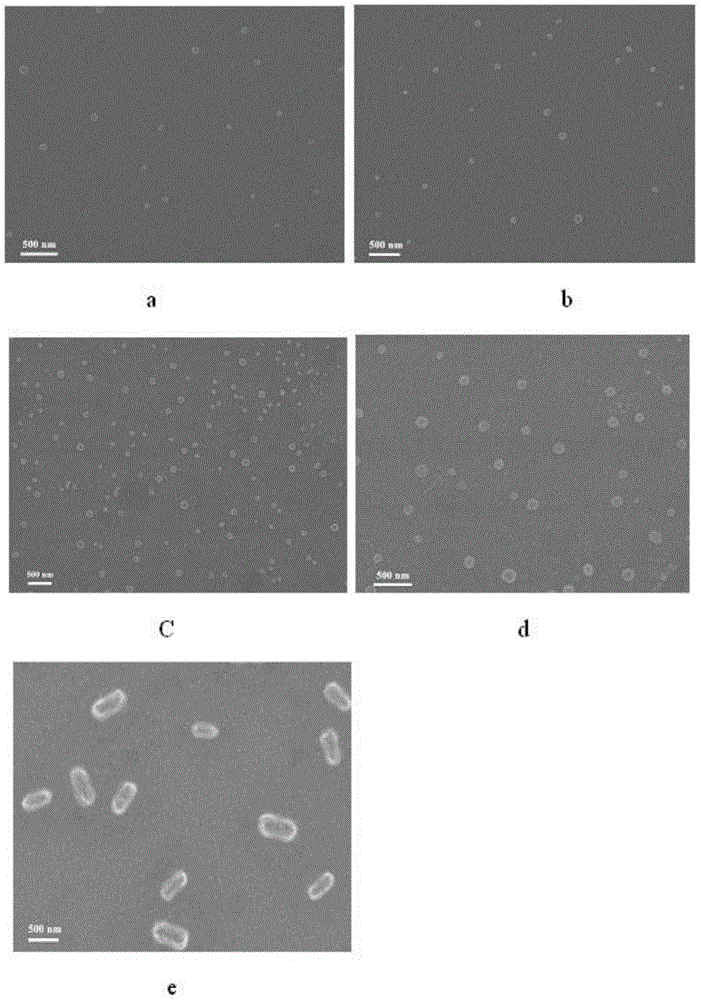
Image
Examples
Embodiment 1
[0041] 1) Synthesis of N,N-bis(acryloyl)cystamine:
[0042] Add 11.6 g of cystamine dihydrochloride into a 250 mL one-necked flask, then add 50 mL of distilled water to stir and dissolve cystamine dihydrochloride. Place the flask in a mixture of ice and water at 0°C; in addition, weigh 8g of solid sodium hydroxide and dissolve it in 20mL of distilled water, add the dissolved sodium hydroxide solution to the single-necked flask at one time, and add 19mL of pre-refined Acryloyl chloride was mixed with 3mL of dichloromethane to form a solution, which was added dropwise into a single-necked flask through a constant pressure dropping funnel. After the dropwise addition was completed within 40 minutes, the reaction was controlled at 25°C for 16h. The product was filtered, washed three times with deionized water, finally recrystallized with ethyl acetate, and dried in a vacuum oven for 24 hours to obtain the product.
[0043] 2) Synthesis of poly(taurine-co-N,N-bis(acryloyl)cystamin...
Embodiment 2
[0051] pH Sensitivity of Reductively Degradable Polyzwitterionic Nanomicelles:
[0052] The reductively degradable polyzwitterionic nanomicelles obtained in Example 1 were respectively placed in buffer solutions of pH=5.0, 6.5, 7.4, and 10.0, and the zeta potential changes were observed with a zeta potential measuring instrument. The results are shown in Figure 5 , wherein the molar ratio of N,N-bis(acryloyl)cystamine:taurine:dodecylamine=1:0.8:0.2; in Figure 5 Among them, when the pH value is 7.4, the solution is weakly alkaline, the amino groups in the micelles are difficult to take up protons, the positive charge is suppressed, and the negatively charged ions in the micelles are dominant, so the zeta potential value is negative. Under acidic conditions at pH 5.0 and 6.5, the amino groups in the micelles take up protons to form ammonium cations, and as time increases, the degree of protonation increases, so the Zeta potential turns into a positive value.
Embodiment 3
[0054] Reduction Sensitivity of Reductively Degradable Polyzwitterionic Nanomicelles:
[0055] The reductively degradable polyzwitterionic nanomicelles obtained in Example 1 were placed in a glutathione solution with a concentration of 10 mM, wherein N,N-bis(acryloyl) cystamine in the nanomicelles: taurine: ten The mol ratio of diamine=1:0.8:0.2, test the particle size change of nano-micelle with laser light scattering instrument at different times, observe reduction responsiveness; The result is as follows Figure 6 Shown, nanomicelle is in the PBS buffer solution that does not contain glutathione (GSH), experiences particle diameter not to change after 24 hours; But nanomicelle is in the glutathione (GSH) solution that contains 10mM, After 12 hours, the particle size became smaller, indicating that some disulfide bonds were broken, and the molecular weight of the copolymer decreased. At this time, the copolymer re-self-assembled to form nanomicelles with smaller particle siz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com