Growth factor controllable slow-releasing system composite multilayer membrane promoting ossification and preparation method thereof
A technology of composite multi-layer film and growth factor, applied in the direction of prosthesis, medical science, etc., can solve the problems of lack of biological activity and cell histocompatibility, lack of biological regulation function, lack of cell-specific recognition sites, etc. Achieving good attracting and receiving ability, improving cell affinity and improving biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Layer-by-layer self-assembly method to construct a composite multi-layer film of growth factor controllable slow-release system that promotes ossification
[0058] 1. Preparation
[0059] S1. Using hexamethylenediamine to treat the surface of the substrate for amination treatment;
[0060] S2. Place the surface-aminated substrate in the heparin / casein solution (negatively charged macromolecule assembly solution) prepared with HEPES, and soak for 15 minutes to make the PU surface adsorb a layer of negatively charged casein and heparin; then move to Rinse 3 times in HEPES solution with a pH value of 7;
[0061] The concentration of casein in the heparin / casein solution is 0.2 mg / mL and the concentration of heparin is 4 mg / mL;
[0062] S3. Place the assembled polyanion electrolyte substrate obtained in S2 in aFGF / collagen solution (positively charged macromolecule assembly solution) and soak for 15 minutes to absorb monolayer collagen and aFGF; then move to H...
Embodiment 2
[0066] Example 2 Regulation of the content of heparin in the composite multilayer film by the number of assembled layers
[0067] The composite multilayer film was prepared according to the method in Example 1, and the content of heparin in the layer-by-layer self-assembly process was determined by the toluidine blue method.
[0068] 1. Determination of the content of heparin loaded on the composite multilayer membrane
[0069](1) Assemble according to Implementation 1. After each layer of heparin is assembled, soak the composite multilayer membrane in 500 μL of toluidine blue buffer, and shake for 30 minutes; the formula of the toluidine blue buffer is: Dissolve 0.2g NaCl, 100uL 36% concentrated hydrochloric acid and 5mg toluidine blue in 1000mL deionized water.
[0070] (2) Add 1mL of n-hexane, shaker and mix well for 20min;
[0071] (3) Remove the organic phase, remove the lower aqueous phase, add to a 96-well plate, and detect the absorbance with a UV spectrophotometer...
Embodiment 3
[0075] Example 3 Regulating the Content of Growth Factors in Multilayer Films by Adjusting the Number of Assembled Layers
[0076] The composite multilayer film was prepared according to the method of Example 1, and the successful construction of the casein / growth factor composite multilayer film was determined by fluorescence tracking after rhodamine-labeled acidic fibroblast growth factor.
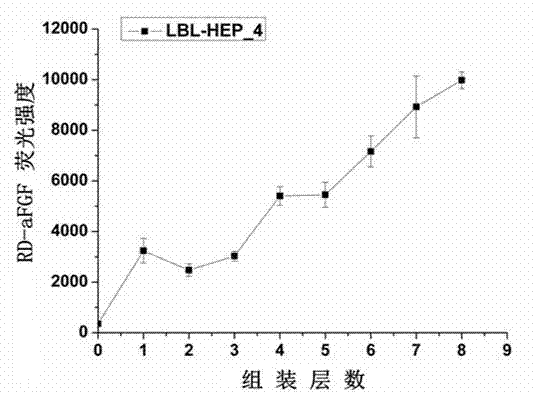
[0077] 1. Use rhodamine (RD) to label acidic fibroblast growth factor (aFGF) to obtain rhodamine-labeled acidic fibroblast growth factor (RD-aFGF).
[0078] 2. RD-aFGF was substituted for unlabeled aFGF, and assembled according to Example 1. After each layer of aFGF was assembled, its fluorescence intensity was detected with a microplate reader.
[0079] 3. Repeat step 2 to complete the construction of the casein / growth factor composite multilayer film, and analyze the relationship between the fluorescence intensity and the number of assembled layers. The results are attached figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com