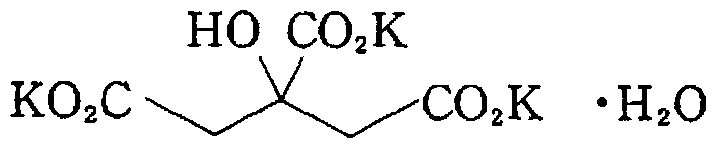
Novel potassium citrate sustained release tablet and preparation method thereof
A technology of potassium citrate and sustained-release tablets, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as potassium citrate sustained-release tablets. Achieve the effect of good drug stability and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
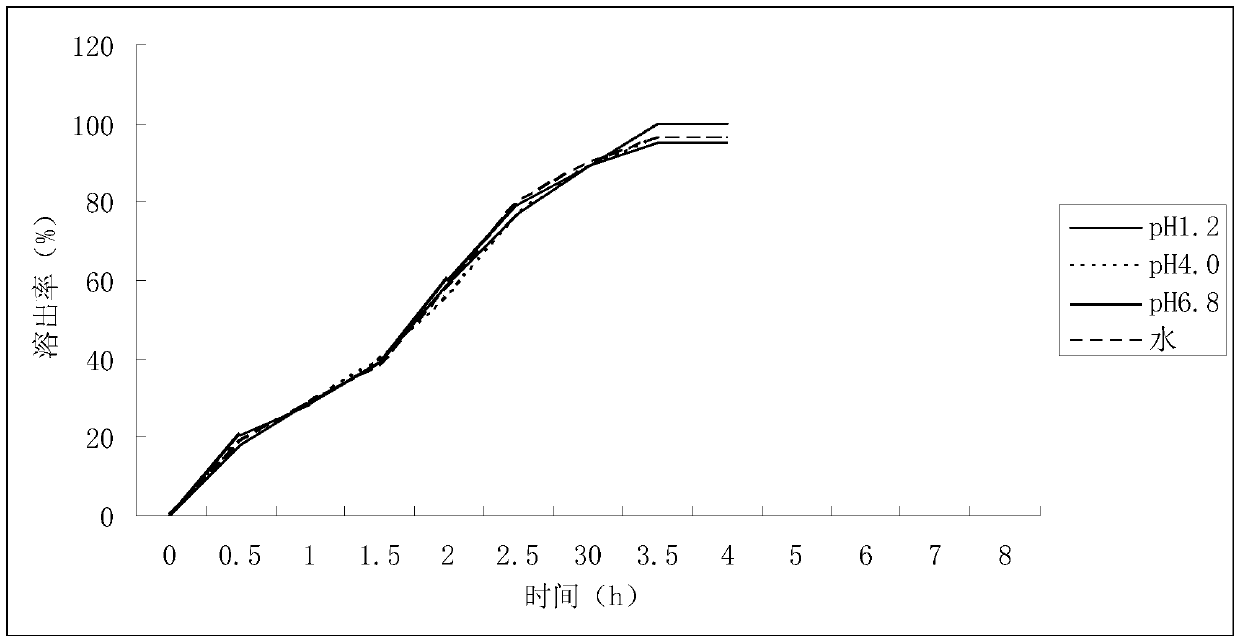
Image
Examples
Embodiment 1
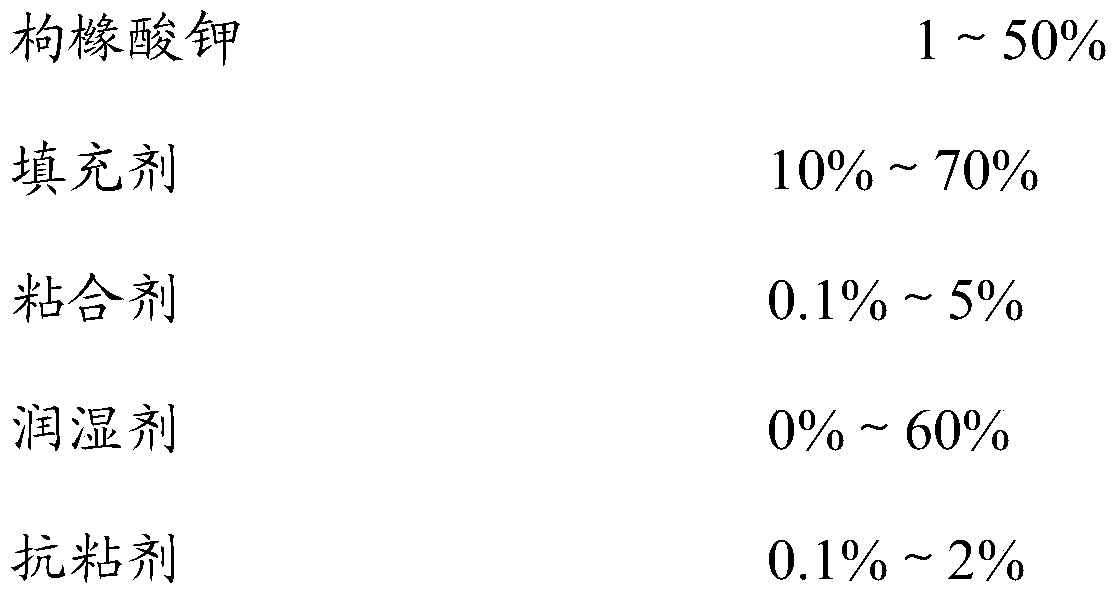
[0036] The percentage in the prescription is the percentage by weight of the component in the whole prescription, and the percentage of lipid material is calculated by the weight of the granules containing the drug, which is the same in the following examples.
[0037]
[0038]
[0039] To prepare potassium citrate extended-release tablets:
[0040] Step 1: Crushing: put potassium citrate, microcrystalline cellulose, hypromellose, and talc into the pulverizer respectively, start the machine, and make the powder into a powder that meets the requirements of an 80-mesh sieve;
[0041] Step 2: Ingredients weighing: check the raw and auxiliary materials sent according to the preparation instruction list, and carry out ingredient weighing;
[0042] Step 3: Granulation: Add lipid material / porogen with wetting agent, heat, stir / homogenize to form lipid liquid, and set aside; Stir the lipid liquid until it reaches the point where it forms a ball and disperses when touched, and t...
Embodiment 2
[0049]
[0050]
[0051] To prepare potassium citrate extended-release tablets:
[0052] Step 1: Crushing: put potassium citrate, starch, hypromellose, and talc into the pulverizer respectively, start the machine, and make the powder material to meet the requirements of 80-mesh sieve;
[0053] Step 2: Ingredients weighing: check the raw and auxiliary materials sent according to the preparation instruction list, and carry out ingredient weighing;
[0054] Step 3: Granulation: Add lipid material / porogen with wetting agent, heat, stir / homogenize to form lipid liquid, and set aside; Stir the lipid liquid until it reaches the point where it forms a ball and disperses when touched, and then it can be discharged;
[0055] Step 4: Drying: Immediately place the granules in an air-heated drying oven, dry, and discharge;
[0056] Step 5: Grain sizing: pass the dried granules through a 20-50 mesh sieve, and the 20-50 mesh sieve is the qualified granule;
[0057] Step 6: Tablet co...
Embodiment 3
[0061]
[0062]
[0063] To prepare potassium citrate extended-release tablets:
[0064] Step 1: Crushing: put potassium citrate, lactose, hypromellose, and talc into the pulverizer respectively, start the machine, and make the powder into a 80-mesh sieve;
[0065] Step 2: Ingredients weighing: check the raw and auxiliary materials sent according to the preparation instruction list, and carry out ingredient weighing;
[0066] Step 3: Granulation: Add lipid material / porogen with wetting agent, heat, stir / homogenize to form lipid liquid, and set aside; Stir the lipid liquid until it reaches the point where it forms a ball and disperses when touched, and then it can be discharged;
[0067] Step 4: Drying: Immediately place the granules in an air-heated drying oven, dry, and discharge;
[0068] Step 5: Grain sizing: pass the dried granules through a 20-50 mesh sieve, and the 20-50 mesh sieve is the qualified granule;
[0069] Step 6: Tablet compression: perform tablet com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com