Amphiphilic block copolymer and preparation method thereof
An amphiphilic block and polymer technology, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and emulsion delivery, etc. Dissolving in supercritical carbon dioxide and other problems, achieving the effects of complete morphology, good drug encapsulation efficiency, and no organic solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
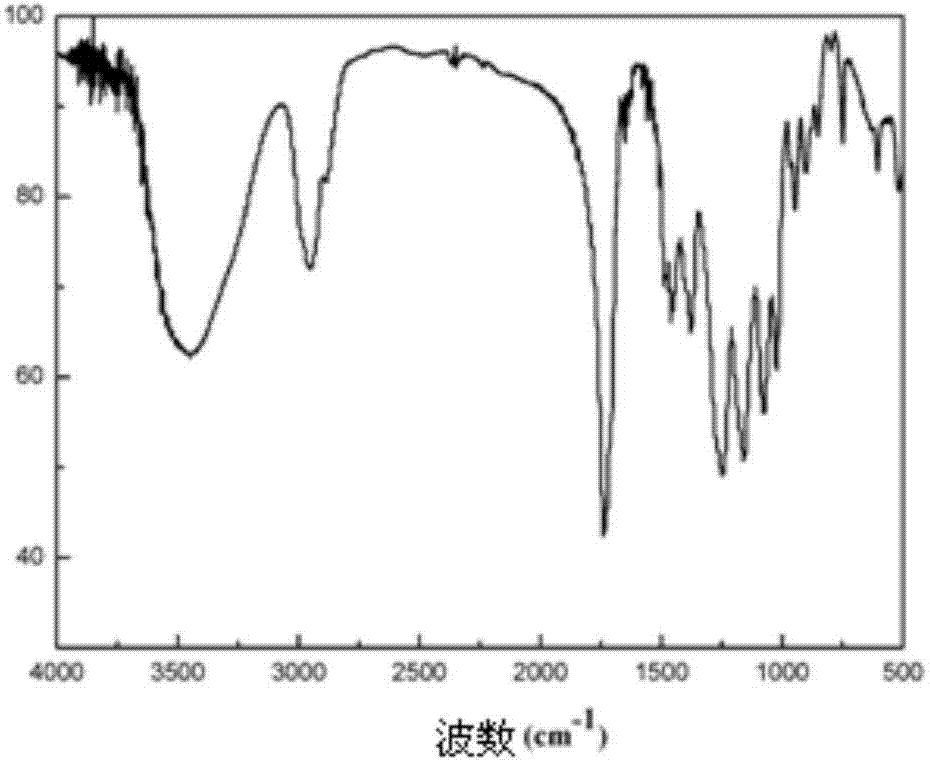
[0025] Example 1: Preparation of an amphiphilic block polymer with one end hydrophilic and one end hydrophilic to supercritical carbon dioxide:
[0026] Step 1, the preparation of vinyl acetate monomer polymerization segment:
[0027] Dissolve 25g vinyl acetate, 2.3g chain transfer agent O-ethyl xanthate propionate and 0.7g azobisisobutyronitrile in 100ml ethanol solution, stir and react at 50 ° C for 10 hours, after the reaction the ethanol solution obtains A vinyl acetate polymer segment with a double bond at the end;
[0028] Step 2, preparation of amphiphilic block polymer:
[0029] Add 25 g of n-butyl methacrylate and 0.7 g of azobisisobutyronitrile to the ethanol solution containing the vinyl acetate polymerized segment in step 1, and stir the reaction at 70 ° C for 16 hours. After the reaction, the organic solvent ethanol is removed by rotary evaporation. Obtain the polymer crude product with amphiphilic properties, dissolve the polymer crude product in 50 ml of dichl...
Embodiment 2
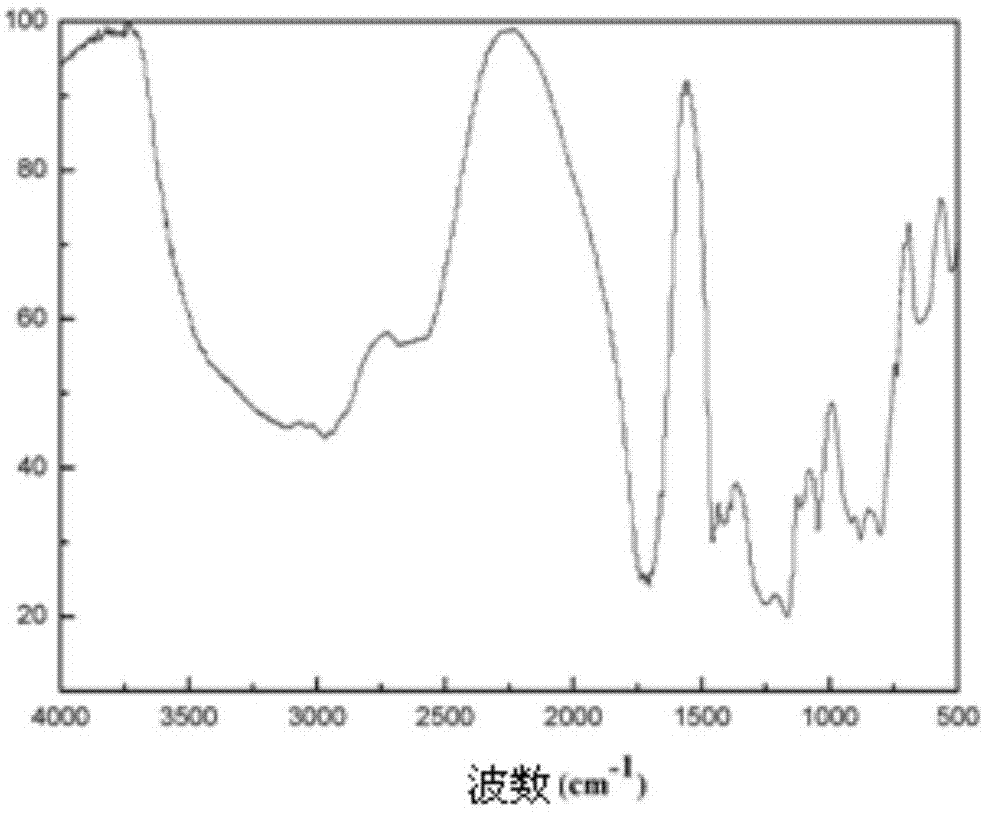
[0030] Example 2: Preparation of an amphiphilic block polymer with one end hydrophilic and one end hydrophilic to supercritical carbon dioxide:
[0031] Step 1, the preparation of vinyl acetate monomer polymerization segment:
[0032] Dissolve 12.5g vinyl acetate, 0.6g O-ethylxanthate ethyl propionate and 0.2g azobisisobutyronitrile in 100ml acetonitrile solution, stir and react at 70°C for 5 hours, after the reaction, the end band is obtained in the acetonitrile solution. Double bond vinyl acetate polymer segment;
[0033] Step 2, preparation of amphiphilic block polymer:
[0034] 37.5 g of hydroxyethyl methacrylate and 0.2 g of azobisisobutyronitrile were added to the acetonitrile solution containing vinyl acetate polymerized segments in step 1, and the reaction was stirred at 90° C. for 10 hours, and the organic solvent acetonitrile was removed by rotary evaporation to obtain The crude polymer product with amphiphilic properties was dissolved in 50 ml of dichloromethane, ...
Embodiment 3
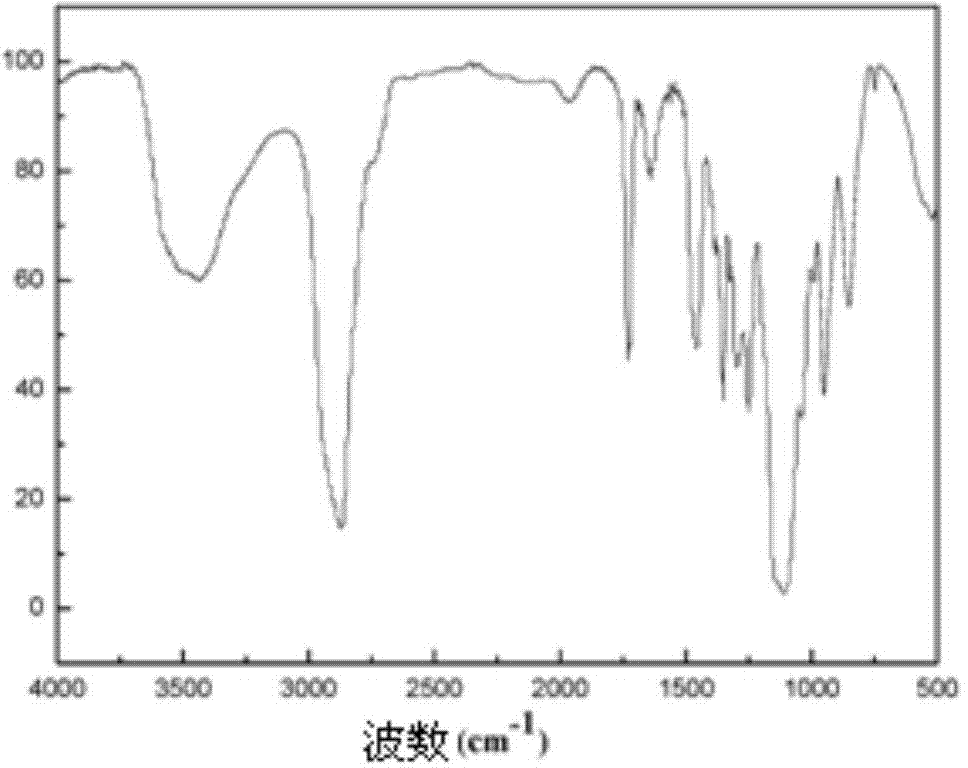
[0035] Example 3: Preparation of an amphiphilic block polymer with one end hydrophilic and one end hydrophilic to supercritical carbon dioxide:
[0036] Step 1, the preparation of vinyl acetate monomer polymerization segment:
[0037] Dissolve 37.5g of vinyl acetate, 0.1g of O-ethylxanthate ethyl propionate and 0.05g of azobisisobutyronitrile in 100ml of 1,4-dioxane solution, stir and react at 90 ° C for 6 hours, the reaction The latter 1,4-dioxane solution obtains a vinyl acetate polymerized segment with double bonds at the end;
[0038] Step 2, preparation of amphiphilic block polymer:
[0039] To the 1,4-dioxane solution containing vinyl acetate polymerized segments in step 1, add 12.5 g polyethylene glycol dimethacrylate (wherein the number of polyethylene glycol monomer segments is 3) and 0.05 g Azobisisobutyronitrile was stirred at 90°C for 12 hours, and the organic solvent 1,4-dioxane was removed by rotary evaporation to obtain a crude polymer product with amphiphilic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com