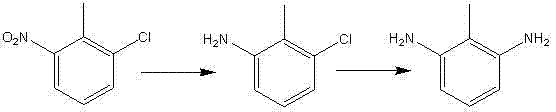
Method for synthesizing 2, 6-diaminotoluene
A technology of diaminotoluene and nitrotoluene, which is applied in the field of synthesizing 2,6-diaminotoluene, can solve the problems of high reaction temperature, equipment corrosion, and low yield, and achieve good reaction selectivity, less by-products, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Reduction reaction: put a certain amount of 2-chloro-6-nitrotoluene 50g, Raney nickel 1g, dicyandiamide 0.05g, methanol 79g in a 250ml autoclave, seal the kettle, stir, nitrogen replacement three times, hydrogen replacement Three times, fill with hydrogen to 0.2MPa, take a sample after reacting at 50°C for 4 hours, until the raw material 2-chloro-6-nitrotoluene is completely reacted; filter the reaction solution to remove the catalyst, and recover methanol under reduced pressure to obtain 3-chloro-2-methanol The crude product of phenylaniline, the crude product obtained 37g of pure 3-chloro-2-methylaniline after vacuum distillation (yield 90%), GC>99%;
[0028] (2) Ammonolysis reaction: 20g of 3-chloro-2-methylaniline, 5g of cuprous chloride, 75g of 25% ammonia water, 2g of urea were put into a 250ml autoclave, the kettle was sealed, stirred, heated to 160°C, and the pressure was 2.3 MPa, take a sample after 20 hours of reaction until the raw material 3-chloro-2-met...
Embodiment 2
[0030] (1) Reduction reaction: drop into 2-chloro-6-nitrotoluene 50g in 250ml autoclave, Raney nickel 1g, dicyandiamide and morpholine compound 0.05g, the weight ratio of dicyandiamide and morpholine is 1 : 0.5, ethanol 79g, seal the kettle, stir, nitrogen replacement three times, hydrogen replacement three times, fill hydrogen to 0.1MPa, take a sample after reacting at 30°C for 4 hours, until the raw material 2-chloro-6-nitrotoluene is completely reacted; The catalyst was removed by filtration, and ethanol was recovered under reduced pressure to obtain a crude product of 3-chloro-2-methylaniline, which was 38 g (yield 92.5%) after vacuum distillation, GC>99%;
[0031] (2) Ammonolysis reaction: 20g of 3-chloro-2-methylaniline, 5g of cuprous oxide, 75g of 10% ammonia solution, 2g of benzaldehyde are put into a 250ml autoclave, the kettle is sealed, stirred, heated to 180°C, and the pressure is 3.0MPa, take a sample after 20 hours of reaction until the raw material 3-chloro-2-me...
Embodiment 3
[0033](1) Reduction reaction: drop 2-chloro-6-nitrotoluene 50g in 250ml autoclave, Raney nickel 1g, dicyandiamide and tributyl phosphate compound 0.05g, dicyandiamide and tributyl phosphate compound The weight ratio is 1:0.5, isopropanol 79g, seal the kettle, stir, replace with nitrogen three times, replace with hydrogen three times, fill with hydrogen to 0.2MPa, react at 40°C for 3 hours and take a sample until the raw material 2-chloro-6-nitrotoluene The reaction was complete; the reaction solution was filtered to remove the catalyst, and the isopropanol was recovered under reduced pressure to obtain the crude product of 3-chloro-2-methylaniline, which was 37 g (yield 90%) after vacuum distillation, GC>99%;
[0034] (2) Ammonolysis reaction: 20g of 3-chloro-2-methylaniline, 5g of copper oxide, 75g of 25% ammonia solution, 2g of urotropine are put into a 250ml autoclave, the kettle is sealed, stirred, heated to 150°C, and the pressure At 2MPa, take a sample after reacting for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com