Liquidambaric acid or ursolic acid nano-granule and preparation method thereof
A Lulutong acid and nanoparticle technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as low bioavailability, and achieve good effects and stable performance. , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] A Passepartout acid nanocapsule particle, which uses Passepartout acid as a core material and rubusoside as a wall material. The amounts of the core material and the wall material are calculated according to the mass ratio, and the core material: wall material is 1:10.
[0067] The above-mentioned preparation method of Passepartout acid nanocapsule particles is prepared by high-energy ball milling, and the steps are as follows:
[0068] Accurately weigh 1g of BEA and 10g of rubusoside, put them in an 80ml grinding bowl, use a planetary high-energy enhanced ball mill (FRITSCH premium line Planetary Mills, PULVERISETTE 7, Germany), select parameters: 80ml embedded grinding bowl with self-locking function, dry Grinding method, 3mm grinding ball, 20g steel ball, rotating speed 1100 rpm, reaction time 7min, drug loading = 1:10, finally got Passepartout acid nanocapsules.
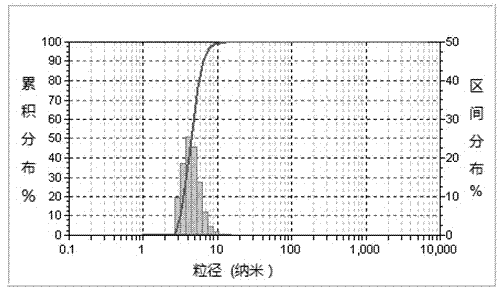
[0069] The particle diameter of the above-mentioned obtained Passepartout acid nanocapsule particles is...
Embodiment 2
[0075] A ursolic acid nanocapsule particle, which uses ursolic acid as a core material and stevioside as a wall material. The amounts of the core material and the wall material are calculated by mass ratio, and the core material: wall material is 1:20.
[0076] The preparation method of above-mentioned a kind of ursolic acid nanocapsule particle adopts high-energy ball milling method to prepare ursolic acid nanoparticle, specifically as follows:
[0077] Accurately weigh 0.5g of URA and 10g of stevioside, and place them in an 80ml grinding bowl, using a planetary high-energy enhanced ball mill (FRITSCH premium line Planetary Mills, PULVERISETTE 7, Germany), selection parameters: 80ml embedded grinding bowl with self-locking function , Dry grinding, 3mm grinding ball, 20g steel ball, rotating speed 900rpm, reaction time 9min, drug loading = 1:20, and finally ursolic acid nanocapsule particles were obtained.
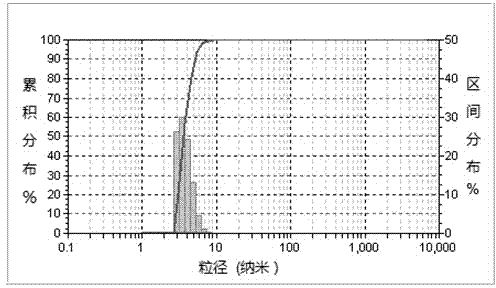
[0078] The particle diameter of the ursolic acid nanocapsule particle...
Embodiment 3
[0083] A Passepartout acid nanocapsule particle, which uses Passepartout acid as a core material and tea saponin as a wall material. The amounts of the core material and the wall material are calculated by mass ratio, and the core material: wall material is 1:30.
[0084] The above-mentioned preparation method of Passepartout acid nanocapsule particles is prepared by an ultrasonic high-speed homogenizer method, which specifically includes the following steps:
[0085] (1) The core material Passepartout acid was added to ethanol, and after ultrasonic treatment and mixing evenly, an ethanol solution of Passepartout acid with a mass percentage concentration of 25% was obtained;
[0086] (2) Dissolving the wall material tea saponin in distilled water, and after ultrasonic treatment and mixing evenly, an aqueous solution of tea saponin with a concentration of 25% by mass is obtained;
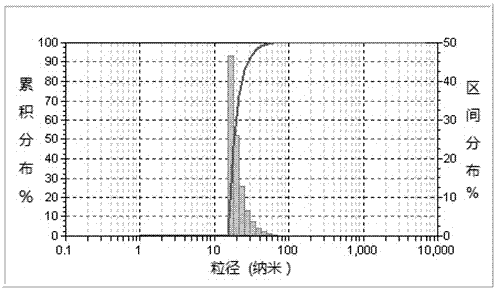
[0087] (3), the Passepartout acid ethanol solution obtained in step (1) and the tea saponin aqueo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility in water | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
| Solubility in water | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com