Structural body
A technology for structures and components, applied in building construction, other chemical processes, transportation and packaging, etc., can solve the problem that the effect of weathering adhesion is not documented, and achieve the effects of excellent weathering adhesion and excellent waterproofing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
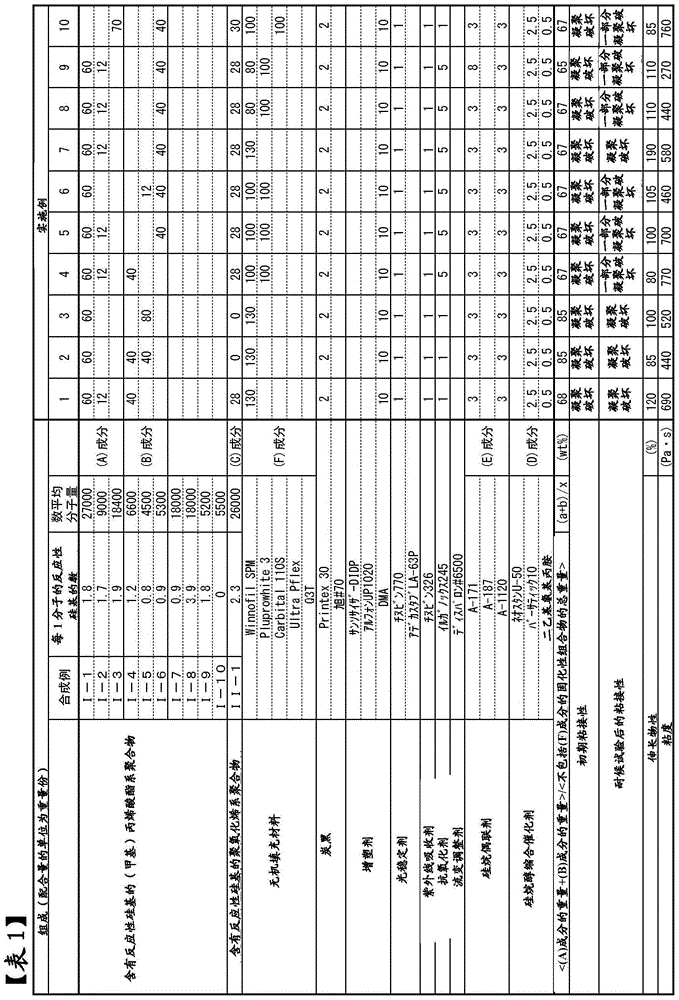
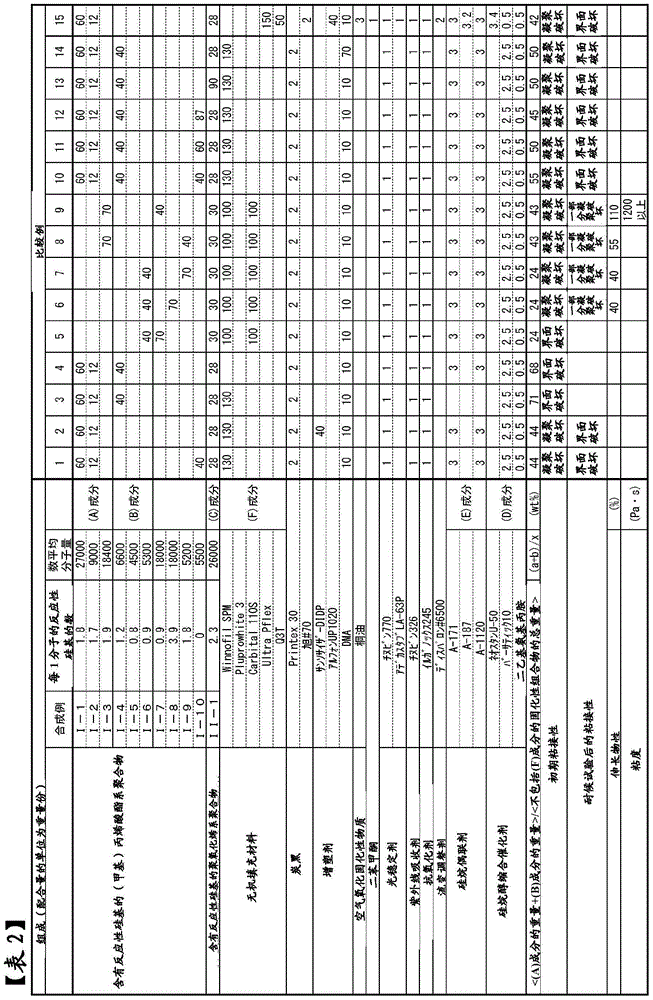
[0285] Hereinafter, specific examples of the present invention will be described together with comparative examples, but the present invention is not limited to the following examples.
[0286] In the following synthesis examples, the "number average molecular weight" was calculated by the standard polystyrene conversion method using GPC. Among them, for the (meth)acrylate polymer of Synthesis Example 1, as a GPC column, a column filled with polystyrene cross-linked gel (shodex GPC K-804; manufactured by Showa Denko Co., Ltd.) was used, and as a GPC solvent, Chloroform was used. For the (meth)acrylate polymers of Synthesis Examples 2 to 6 and Comparative Synthesis Examples 7 to 10 and the polyoxyalkylene polymers of Synthesis Example 11, HLC-8120GPC manufactured by Tosoh Corporation was used as a liquid delivery system. TSK-GEL Type H manufactured by Tosoh Corporation was used, and THF was used as a solvent for measurement.
[0287] In the following synthesis examples, "the ...
Synthetic example 1
[0289] Under nitrogen atmosphere, add CuBr (1.09kg), acetonitrile (11.4kg), n-butyl acrylate (26.0kg) and 2,5-dibromodiethyl adipate (2.28kg) in 250L reactor, at 70 Stir at ~80°C for about 30 minutes. Pentamethyldiethylenetriamine was added thereto to start the reaction. After 30 minutes from the start of the reaction, n-butyl acrylate (104 kg) was continuously added over 2 hours. During the reaction, pentamethyldiethylenetriamine was appropriately added so that the internal temperature was 70°C to 90°C. After 4 hours from the start of the reaction, volatile matter was removed by heating and stirring at 80° C. under reduced pressure. Acetonitrile (45.7 kg), 1,7-octadiene (14.0 kg), and pentamethyldiethylenetriamine (439 g) were added thereto, and stirring was continued for 8 hours. The mixture was heated and stirred at 80° C. under reduced pressure, and the volatile matter was removed.
[0290] Add toluene to the concentrate to dissolve the polymer, then add diatomaceous e...
Synthetic example 2
[0296]In the isobutanol solution of the following monomer mixture heated to 105°C, a solution in which 2,2'-azobis(2-methylbutyronitrile) was dissolved as a polymerization initiator was dropped over 4 hours, and then After performing "post-polymerization" for 1 hour, the solvent was distilled off, and the (meth)acrylate polymer [I-2] which has a reactive silicon group was obtained. The number average molecular weight was 9000. pass 1 The H-NMR analysis determined the average number of silyl groups introduced per one molecule of the polymer, and it was about 1.7.
[0297] Methyl methacrylate: 46.5 parts by weight, n-butyl acrylate: 28.6 parts by weight, stearyl methacrylate: 20.1 parts by weight, γ-(methacryloxypropyl)trimethoxysilane: 4.8 parts by weight , 2,2'-Azobis(2-methylbutyronitrile): 3.2 parts by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com