Monoclonal antibody for detecting porcine C-reactive protein (CRP)
A monoclonal antibody and reactive protein technology, which is applied in the field of immunoassay, can solve the problems of lack of effective detection methods and achieve the effects of simple and efficient preparation methods, reduced drug residues, and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The amplification of embodiment 1 pig CRP gene and the construction of expression vector
[0047] 1. Design and synthesis of primers
[0048] According to the nucleotide sequence of the GenBank number (NM_213844.2) in the database in NCBI, a pair of primers containing the main coding region of the CRP gene were designed using DNAStar and Primer5.0 software. The upstream primer P1 has an NdeI restriction site, and the downstream primer P2 has a There is an Xhol restriction site, and the amplified fragment between the upstream and downstream primers includes 612 bases in the main coding region of the CRP gene.
[0049] Upstream primer P15′-cttcatatgcagacagacatgatcggaaaggcc-3′
[0050] (SEQ ID NO: 1)
[0051] Downstream primer P25'-tgactcgagttagggccacagctggggcttgacatacac-3' (SEQ ID NO: 2)
[0052] 2. Extraction of pig total RNA Take 50-100 mg of pig liver and add it to a 1.5 ml EP tube, first add 300 μL Trizol (Invitrogen), grind it with a grinding rod until it becomes ...
Embodiment 2
[0110] Example 2 Induced expression of recombinant protein and renaturation and purification of expressed protein
[0111] 1. Induced expression of recombinant protein and extraction of inclusion bodies
[0112] (1) Transform the expression plasmid pET21a-pigCRP into BL21(DE3), and after culturing for 12 hours, inoculate the monoclonal colony into 100mL Amp + In LB medium, shake slowly in a shaker for 8-10 hours, and then use it as a mother solution;
[0113] (2) Inoculate 1%-5% mother solution into 3mL culture medium, culture on a shaker at 37°C until OD600 reaches 0.4-0.6, set empty bacteria and empty plasmid controls, and rotate at 200rpm;
[0114] (3) The mother solution is transferred to 2L of Amp according to the inoculation volume of 1%. + In LB medium, 37°C, 180rpm, cultivate to OD value of 0.4-0.6, add IPTG to a final concentration of 1mM, 37°C, 160-180rpm, continue to cultivate for 5h;
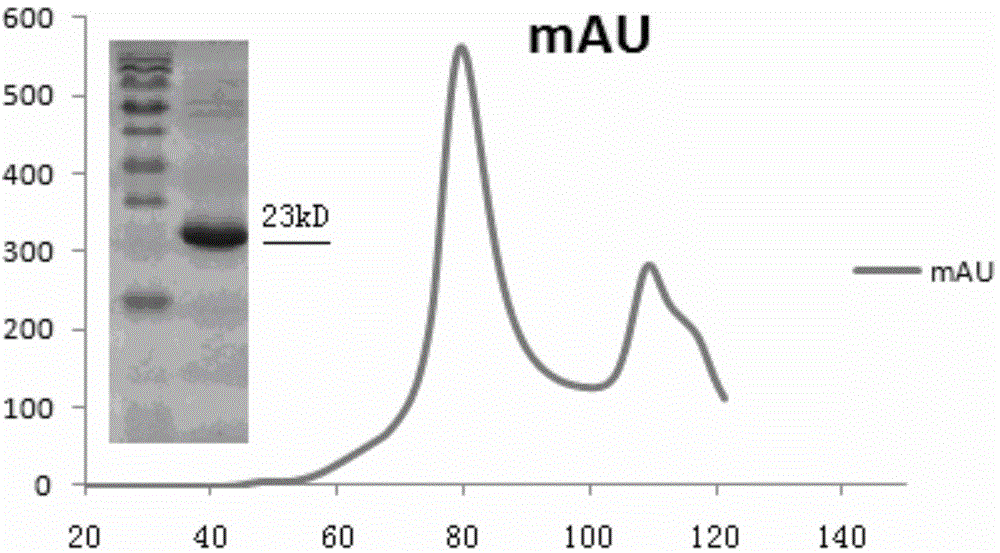
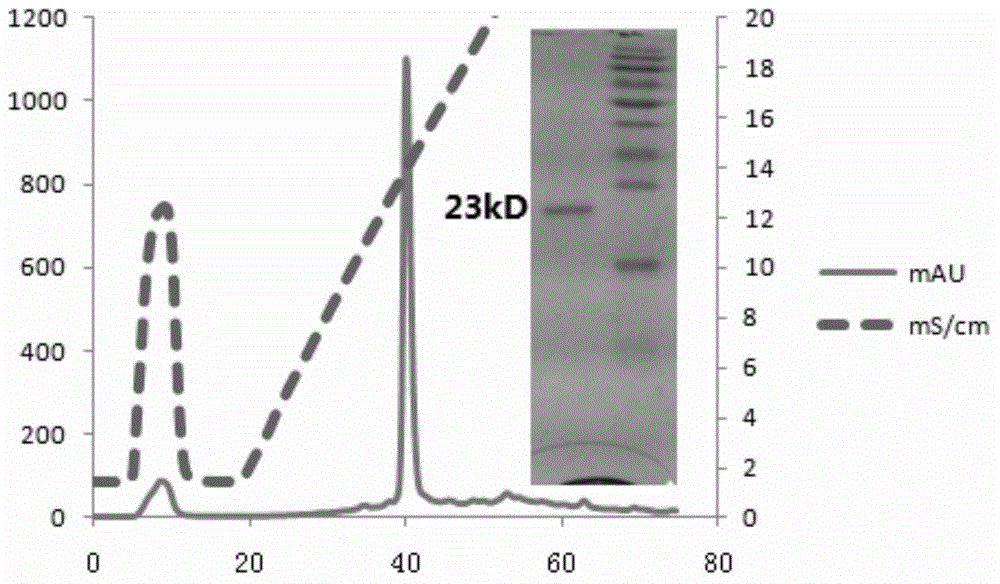
[0115] (4) Harvest bacteria (the following steps are all carried out in ice),...
Embodiment 3
[0140] The preparation of embodiment 3 monoclonal antibody
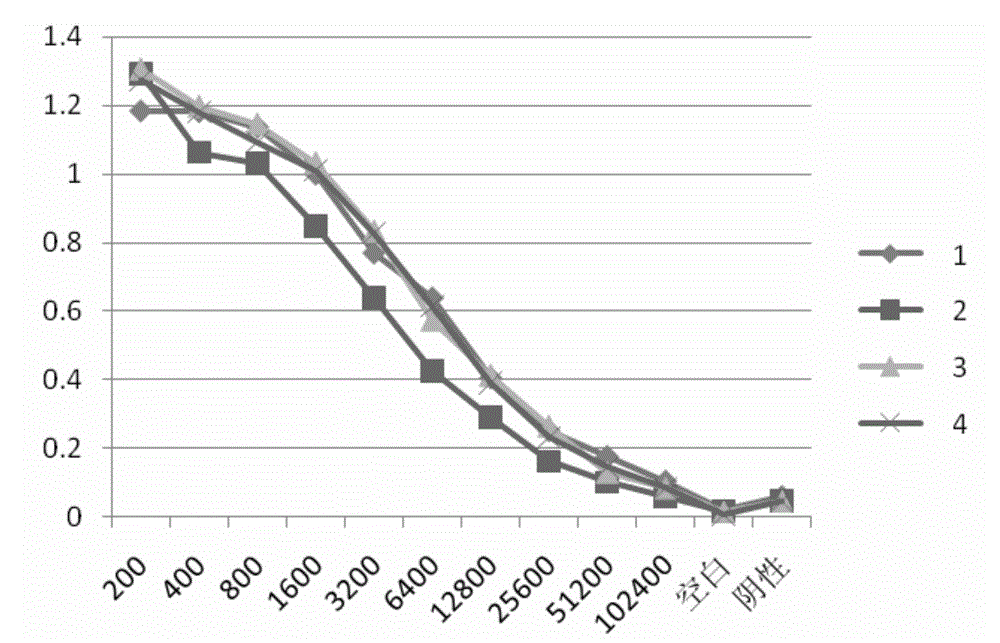
[0141] 1. The porcine CRP protein obtained in Example 2 was used for the determination of antibody titer in immunized mice, and 4 SPF BALB / c female mice were immunized subcutaneously for the first time in the amount of 60 μg protein / mouse, numbered as: 1, 2 , 3, 4. After five subcutaneous booster immunizations (immunization dose: 30 μg protein / mouse), blood was collected from the orbits of the mice to measure the serum titer. Results (see image 3 ) No. 4 mouse had the highest serum antibody titer and was used for cell fusion experiments.
[0142] 2. Cell fusion experiment Take mouse splenocytes and SP2 / 0 cells, and use PEG method for fusion. The fused cells were selected and cultured with semi-solid medium (containing HAT).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com