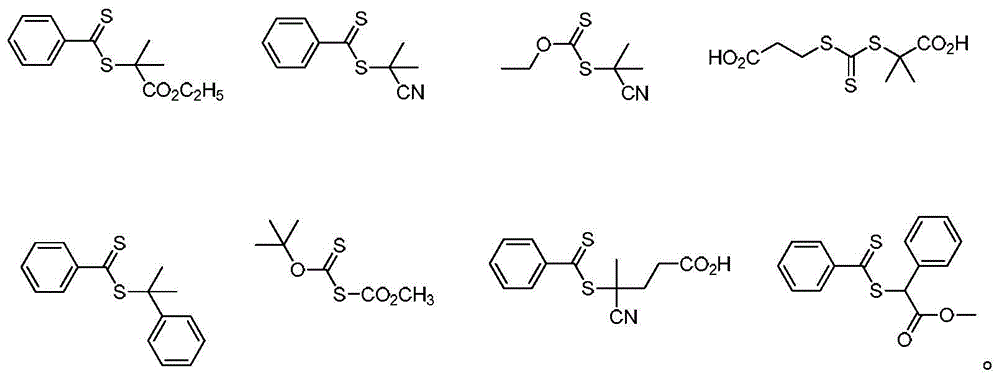
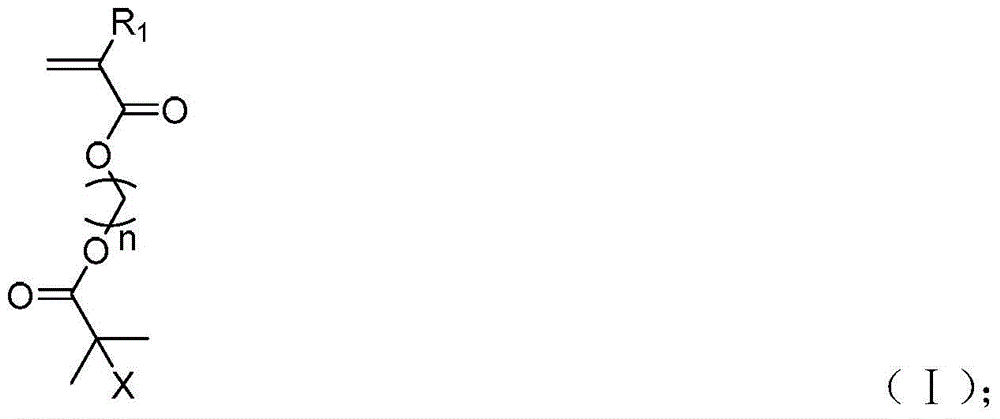Acrylate type polychain transfer agent, its preparation method and its application in the preparation of columnar polymer brushes
A columnar polymer brush and acrylate-type technology, which is applied in the application field of preparing columnar polymer brushes, can solve the problems of complex structure, cumbersome synthesis process, and limited application of small molecule thioester compounds, and achieve simple and efficient introduction, huge Application prospects, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 3.0 g of methacrylate-2-(2-bromo-2-methylpropionyl)oxyethyl ester (BIEM), 11.7 mg of chain transfer agent dithiobenzoic acid-2-phenylpropan-2-ester ( CDB), 2.35mg AIBN, and 10mL anisole were added to the reaction flask, the molar ratio of BIEM, AIBN, and CDB was 250:0.33:1, reacted at 70°C for 24 hours, dissolved and precipitated in 200mL n-hexane for 3 times to remove small molecules Body, vacuum-dried to get light pink homopolymer main chain polymethacrylate-2-(2-bromo-2-methylpropionyl)oxyethyl ester (PBIEM), yield was 40%, polymerization main chain NMR calculation The degree of polymerization is 185, the SEC number average molecular weight is 19.4 kDa, and the molecular weight distribution is 1.27.
[0055] Add 0.25g of PBIEM, 0.515mL of stannous octoate, 354.5mg of benzothiodithioperoxyanhydride, 40μL of ethyl 2-bromoisobutyrate and 20mL of toluene into the reaction flask, and pass argon for 0.5h. Deoxygenated (127mg) cuprous bromide / (1.11mL) PMEDTA was added into...
Embodiment 2
[0058] Other polymerization conditions are the same as in Example 1, except that the PBIEM-g-PtBMA obtained in 1 is used to continue to synthesize block side chains. The monomer used is oligoethylene glycol methacrylate (OEGMA), and the specific steps are as follows: add 1.111g OEGMA, 20mg PBIEM-g-PtBMA, 0.2436mg AIBN and 10mL anisole into the reaction flask, and react at 60°C 27h, after the reaction was over, use 200mL of methanol to precipitate 3 times to remove the monomer to obtain polymethacrylate-2-(2-bromo-2-methylpropionyl)oxyethyl ester-g-(polymethacrylate tert-butyl -b-polyethylene glycol methacrylate) PBIEM-g-(PtBMA-b-POEGMA), the yield is 25%, the side chain polymerization degree calculated by NMR is 62.5, and the SEC number average molecular weight is 896.5 kDa, molecular weight distribution is 1.10.
Embodiment 3
[0060] Other polymerization conditions are the same as in Example 1, except that the polymerized monomer for the side chain is OEGMA, and the polymethacrylic acid-2-(2-bromo-2-methylpropionyl)oxyl group of the homopolymerized side chain is synthesized. Ethyl-g-polyethylene glycol methacrylate (PBIEM-g-POEGMA) was reacted at 60°C for 24h. After the reaction, the monomer was removed by precipitation with 150mL ether for 3 times. The yield was 37%. The degree of side chain polymerization calculated by NMR is 50, the SEC number average molecular weight is 434.2kDa, and the molecular weight distribution is 1.27.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com