Horseradish tree leaf tablet and preparation method thereof
A technology of Moringa leaf and Moringa leaf powder, which is applied in the direction of pill delivery, pharmaceutical formula, and medical preparations of non-effective ingredients, etc., can solve the problem that patients are not a good treatment method, and achieve easy swallowing and treatment Good effect, the effect of regulating intestinal flora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
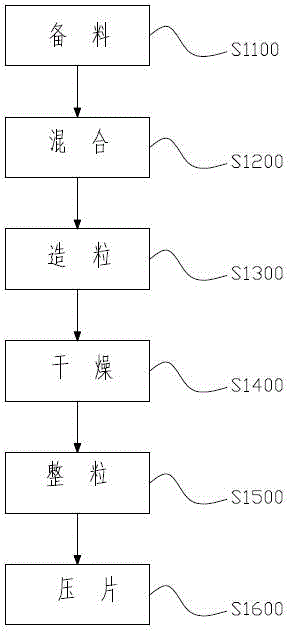
[0038] On the other hand, the invention provides a kind of preparation method of Moringa leaf agent, figure 1 A schematic flow diagram of the preparation method of the Moringa oleifera leaf preparation of the present invention is shown. Such as figure 1 As shown, the method includes the following steps:
[0039] 1) Material preparation step S1100: prepare the following raw materials, the percentage by weight of which is: Moringa oleifera leaf powder 50%-70%, fructo-oligosaccharide 27%-48%, hypromellose aqueous solution 1.5%-2%, stearin Magnesium acid 0.5% ~ 1%;
[0040] 2) Mixing step S1200: putting Moringa oleifera leaf powder and fructooligosaccharide into a blender for uniform mixing, the mixing time is 40 minutes, and the mixture is obtained after thorough mixing;
[0041] 3) Granulation step S1300: adding a hypromellose aqueous solution with a concentration of 1.5% to 2% to the mixture and putting it into a swing granulator for granulation to obtain wet granules, where...
Embodiment 1
[0054] At first prepare the following raw materials, choose 3 mature young Moringa leaves, and the particle size obtained through ultrafine grinding is 200 mesh ultrafine powder, preparation materials: Moringa leaf powder 50%, fructooligosaccharide 48%, hypromellose 1.5% plain aqueous solution, 0.5% magnesium stearate;
[0055] Moringa leaf powder and fructooligosaccharides were dropped into the blender and mixed uniformly, and the mixing time was 40 minutes to obtain the mixture;
[0056] Add 1.5% hypromellose aqueous solution to the mixture and put it into a swing granulator for granulation to obtain wet granules with a screen mesh of 14 mesh; then place the wet granules in a drying oven at 55°C. Drying in the box, the drying time is 4 hours, and the drying end point is that the moisture content of the granules is reduced to 3%. Add 0.5% magnesium stearate, mix evenly, and send it into a tablet machine for tableting, and the net content of the tablet obtained is 0.5g; final...
Embodiment 2
[0058] First prepare the following raw materials, select 4 mature young Moringa leaves, and superfinely pulverize them into 120-mesh ultrafine powder, Moringa leaf powder 60%, fructooligosaccharides 37%, hypromellose aqueous solution 2%, hard Magnesium fatty acid 1%;
[0059] Moringa leaf powder and fructooligosaccharides were dropped into the blender and mixed uniformly, and the mixing time was 40 minutes to obtain the mixture;
[0060] Add 2% hypromellose aqueous solution to the mixture and put it into a swing granulator for granulation to obtain wet granules with a screen mesh of 15 mesh; The drying time is 4.5 hours, and it is advisable to reduce the moisture content of the granules to 3.5% at the end of drying; then granulate the dried granules, and the mesh number of the granules is 14 mesh, and then add 1% stearic acid to the dried granules Magnesium, mixed uniformly, sent into tablet press for tableting, the net content of the obtained tablet is 0.5g; finally the tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com