Glucose derivatives of glaucocalyxin A as well as preparation method and application of glucose derivatives
A technology of glucose derivatives and blue calyxine, which is applied in the field of medical invention and can solve the problems of low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
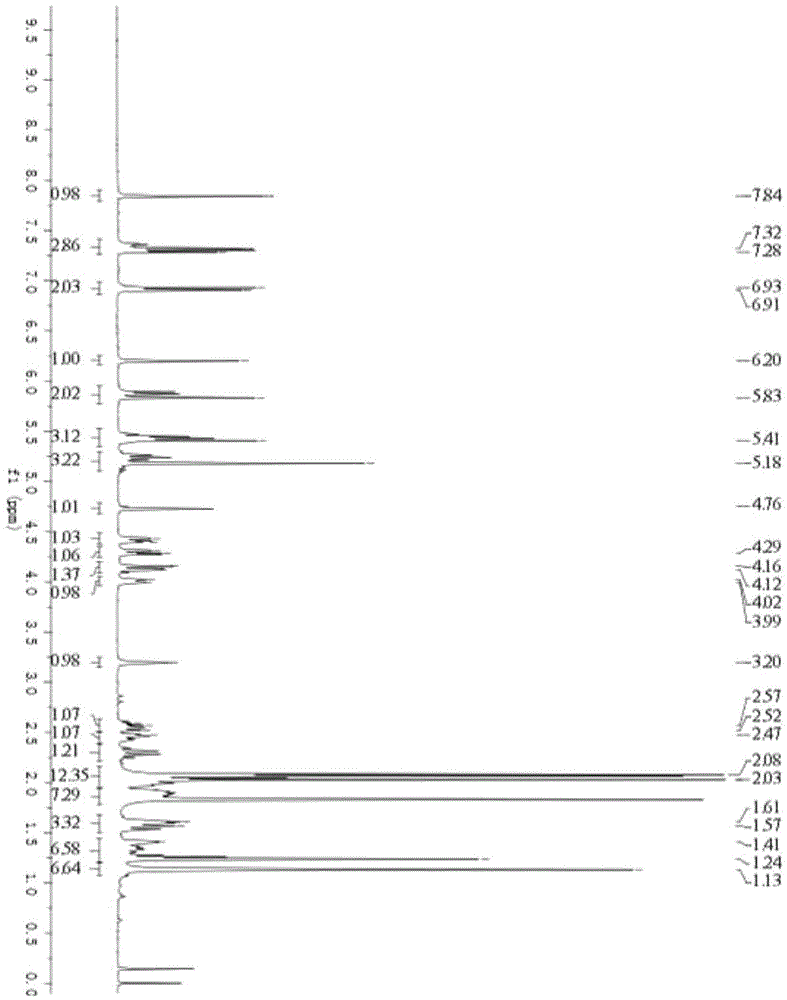
[0072] Embodiment 1: the preparation of the cyanine A glucose derivative Ia, Ib, Ic containing triazole group
[0073] The reaction formula is as follows:
[0074]
[0075] 1.1: Preparation of fully acetylated glucose
[0076] Weigh 8.287g of anhydrous sodium acetate and place it in a crucible, adjust the temperature so that it loses all the water, transfer it to a mortar immediately after cooling, add dry glucose (10.636g, 590mmol), mix well and transfer it to 250mL Add 150 mL of acetic anhydride to the round bottom flask, adjust the temperature to 100°C, and react for 2-3 hours. After cooling to room temperature, the reaction solution was poured into ice water, and a white solid precipitated out. After suction filtration, it was washed with 5 times the amount of distilled water to obtain peracetylated glucose. After drying, 17.707 g of white solid was obtained, with a yield of 77%.
[0077] 1.2: Preparation of fully acetylated bromoglucose
[0078] Take the compound (4...
Embodiment 2
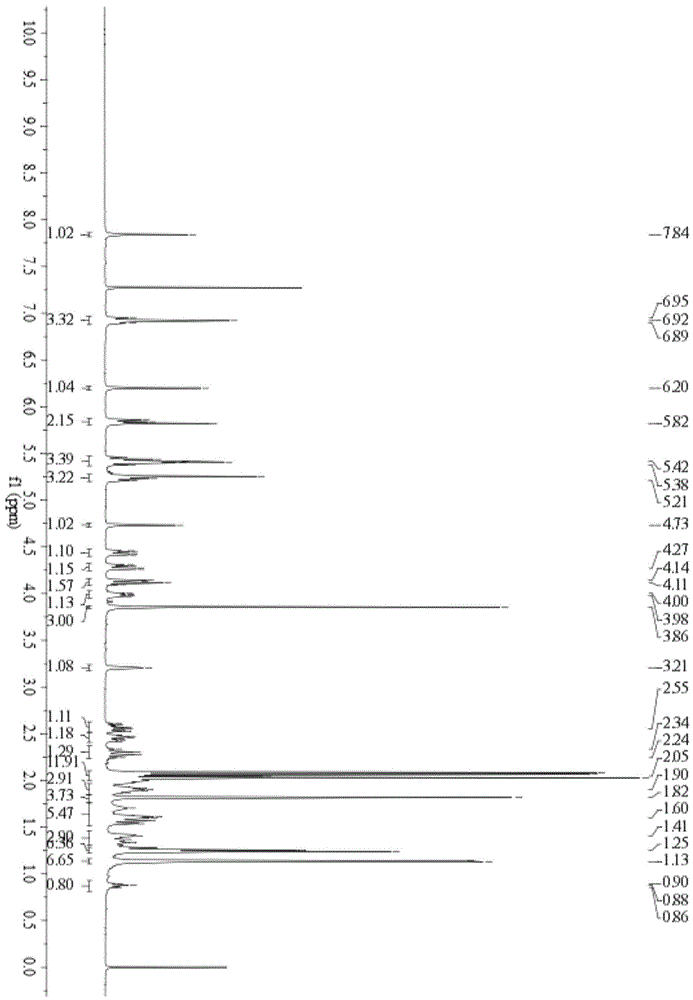
[0108] Embodiment 2: the preparation of the cerulein derivative IIa of glucose sugar ester
[0109] The reaction formula is as follows:
[0110]
[0111] 2.1: Preparation of 2-(4-formyl-2-methoxy-phenoxy)-acetic acid
[0112] Take chloroacetic acid (4.822g, 51.3mmol) and add an appropriate amount of saturated potassium carbonate solution to adjust the pH value of the solution to 9 to dissolve all the samples, and transfer it to a constant pressure dropping funnel for later use. Take 3-methoxy-4-hydroxybenzaldehyde (5.198g, 34.2mmol) in a 250mL bottom flask, add an appropriate amount of 5% sodium hydroxide solution to adjust the pH value to about 9, stir to dissolve all the samples, adjust the constant pressure drop Liquid funnel, transfer all the samples to the round bottom flask within 30min, then add 321mg of potassium iodide, reflux at 110°C for 5h, cool to room temperature, adjust the pH value of the solution to 1 with concentrated hydrochloric acid, precipitate out, f...
Embodiment 3
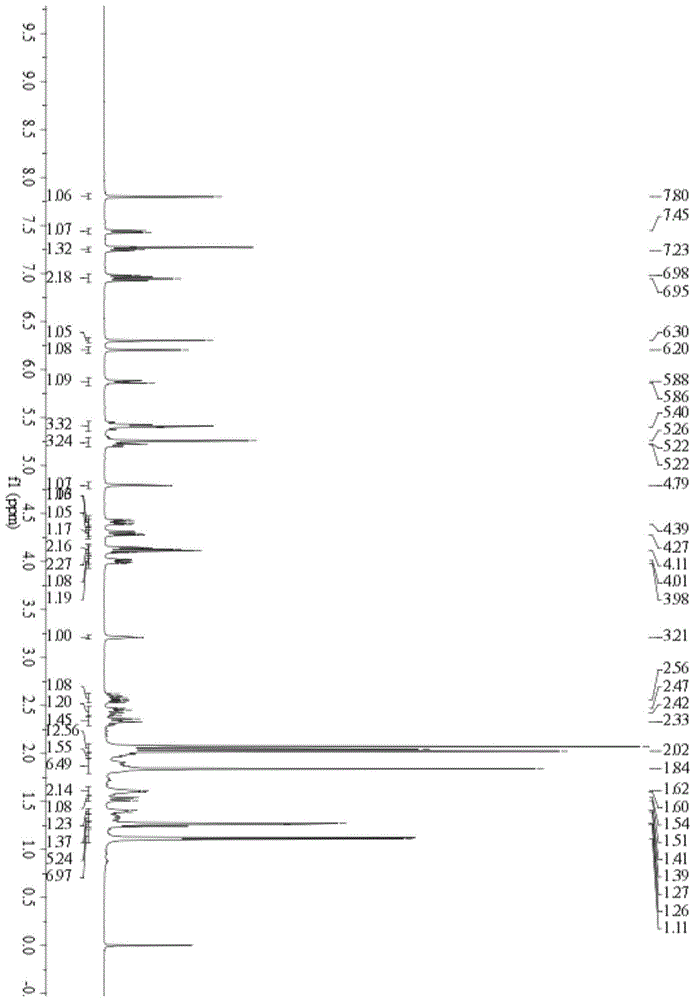
[0118] Embodiment 3: the preparation of the cerulein derivatives IIb and IIc of glucoside
[0119] The reaction formula is as follows:
[0120]
[0121] 3.1: Preparation of peracetylated glucosides substituted by benzaldehyde
[0122] 3.1.1: Take the fully acetylated bromoglucose (2.498g, 6.09mmol) and 3-methoxy-4-hydroxybenzaldehyde (0.926g, 6.09mmol) obtained in 1.2 of Example 1 in a 100mL round bottom flask , add 25mL of dichloromethane to dissolve all the samples, then add TBAB (1.961g, 6.09mmol) and 20mL of saturated potassium carbonate solution, react at 60°C for 5h, cool to room temperature, transfer the reaction solution into a separatory funnel, add Dichloromethane 50mL, washed with distilled water 50mL×3, saturated ammonium chloride 50mL×3, saturated brine, anhydrous MgSO 4 Drying, filtration, concentration under reduced pressure, separation and purification by column chromatography, eluting with petroleum ether / ethyl acetate 3:1, gave 1.758 g of white powdery s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com