Sanduryl boron compound and its preparation method and use
A compound and nitrogen heterocyclic compound technology, applied in the field of fluorescent devices, can solve the problems of cumbersome data reading, low spatial resolution, unsuitable miniaturization, etc., achieve wide response temperature range, high sensitivity, and avoid uneven concentration distribution the effect of interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
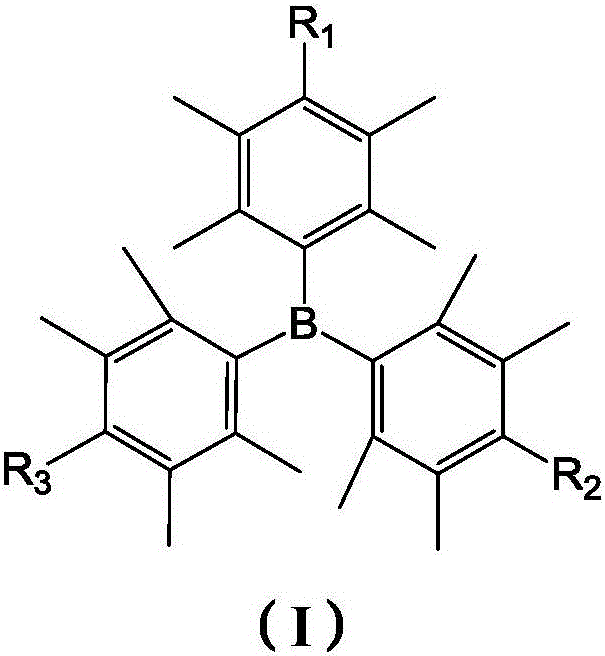
[0048] Preparation of tris(2,3,5,6-tetramethyl-4-morpholinophenyl)borane (MPB)
[0049]
[0050] a) Dissolve 13.4 g of durene (compound 1) in 25 ml of chloroform, add 2 equivalents of bromine, react for 2 hours, and wash with water; obtain white solid compound 2 (26.0 g, 90%).
[0051] b) Dissolve 10g of p-dibromotetramethylbenzene (compound 2) in 300ml of degassed ether, protect with argon, cool down to -78°C, add 1.2 equivalents of n-butyllithium and stir, gradually raise the temperature to 0°C, then cool down again to -78°C. Add 0.3 equivalent of boron trifluoride diethyl ether, return to normal temperature and continue to react for 2 hours. After the reaction, it was washed with water, and purified by silica gel column using petroleum ether as developing solvent. Compound 3 (3.3 g, 45%) was obtained as a white solid.
[0052] c) 4.5 equivalents of sodium tert-butoxide (NaOtBu), 50 ml of toluene, 0.09 equivalents of 2,2'-bisdiphenylphosphino-1,1'-binaphthyl (BINAP) an...
Embodiment 2
[0054] Preparation of 4,4'-(((4-bromo-2,3,5,6-tetramethylphenyl)boron)bis(2,3,5,6-tetramethyl-4,1-benzene))bis Morpholine (BrMPB)
[0055]
[0056] a) Dissolve 13.4 g of durene (compound 1) in 25 ml of chloroform, add 2 equivalents of bromine, react for 2 hours, and wash with water; obtain white solid compound 2 (26.0 g, 90%).
[0057] b) Dissolve 10g of p-dibromotetramethylbenzene (compound 2) in 300ml of degassed ether, protect with argon, cool down to -78°C, add 1.2 equivalents of n-butyllithium and stir, gradually raise the temperature to 0°C, then cool down again to -78°C. Add 0.3 equivalent of boron trifluoride diethyl ether, return to normal temperature and continue to react for 2 hours. After the reaction, it was washed with water, and purified by silica gel column using petroleum ether as developing solvent. Compound 3 (3.3 g, 45%) was obtained as a white solid.
[0058] c) 3 equivalents of sodium tert-butoxide (NaOtBu) in 50 ml of toluene, 0.06 equivalents of ...
Embodiment 3
[0060] Fabrication of MPB's PEG-4000 solid-state polymer temperature-fluorescent device
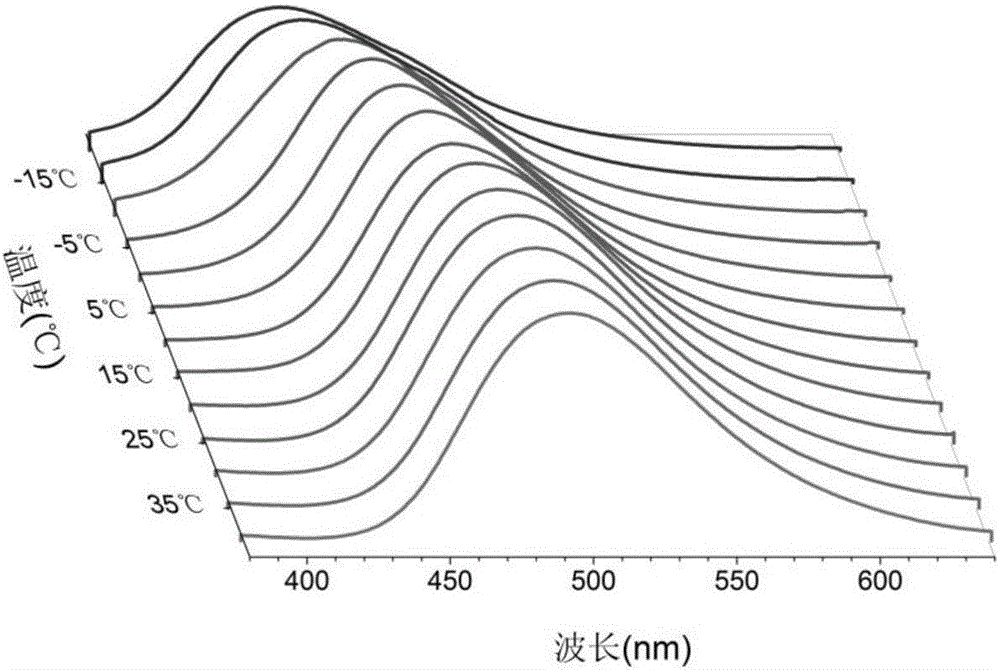
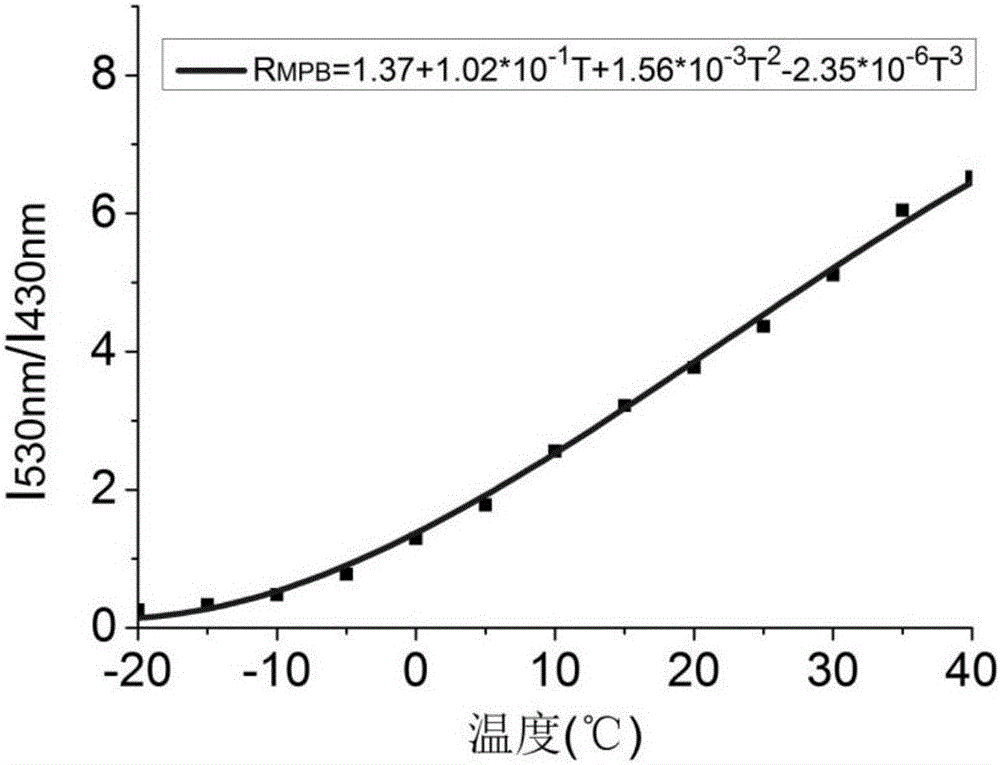
[0061] Polymer solid-state polymer temperature-fluorescent device with an action range of -20°C to 40°C, the compound used is the tris(2,3,5,6-tetramethyl-4-morpholine benzene) boron (MPB ). The temperature-fluorescence device is obtained through the response of its two fluorescent excited states to the viscosity of the environment, and the viscosity of the PEG4000 polymer changes with the change of the environment temperature.
[0062] (1) The preparation concentration is 1×10 -3 mol / liter carbon dichloride solution of MPB compound;
[0063] (2) Take 1ml of the solution and drop it into 12.7g (room temperature 10cm 3 ) The melted PEG4000 macromolecule is mixed evenly, and the dichloromethane is removed in a vacuum oven, and the temperature is kept above the melting point of PEG4000;
[0064] (3) At this time, the concentration of MPB in PEG4000 polymer is 1×10 -4 mol / cubic decimeter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com