Preparation method of (R)-praziquantel
A technology of L-praziquantel and intermediates, which is applied in the field of preparation of L-praziquantel (praziquantel), can solve the problems of high cost, cumbersome operation, and high labor intensity, and achieve the effect of low cost and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 recombinant D-amino acid oxidase
[0038] Inoculate a single colony of recombinant Escherichia coli containing the D-amino acid oxidase gene from a glycerol tube or transformation plate into 4mL liquid LB medium containing (100ug / mL) ampicillin resistance, and activate overnight at 37°C for 12-16 hours , the culture obtained after activation was transferred to 100mL liquid LB medium containing (100ug / mL) ampicillin resistance with 2% inoculum, and cultured at 37°C and 220rpm until OD 600 When the value reaches about 0.6, add the inducer isopropyl-β-D-thiogalactopyranoside to a final concentration of 0.8mmol / L, and continue culturing overnight at 30°C. Collect the cells by centrifugation (4°C, 5000 rpm, 15 min), and suspend the cells with 10 mL of phosphate buffer (100 mM, pH 7.0). Place the cell suspension in an ice bath for 10 minutes, then centrifuge (4°C, 12000rpm, 15min), pre-freeze the supernatant at -20°C overnight, and then freez...
Embodiment 2
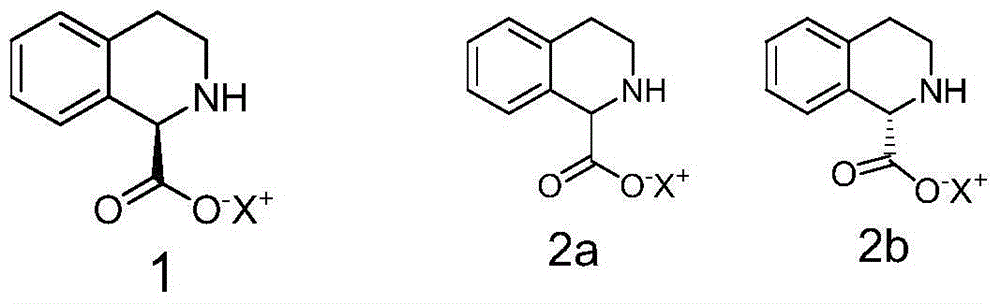
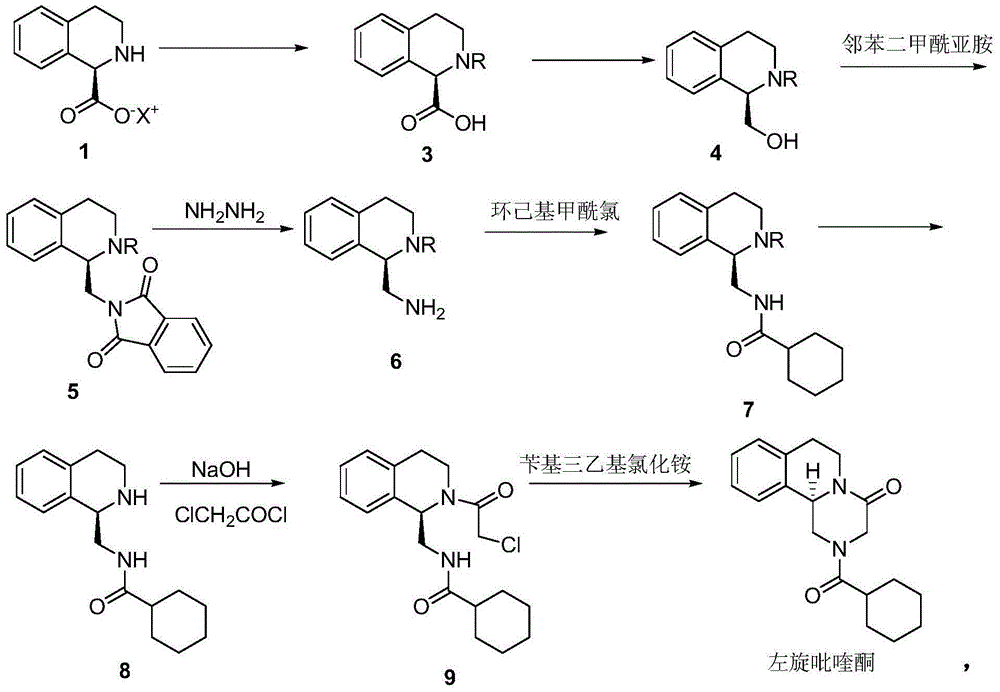
[0039] Preparation of embodiment 2 intermediate 1-(R)-tetrahydroisoquinoline formic acid ammonium salt
[0040] Dissolve 1.77g (0.01mol) DL-tetrahydroisoquinoline-1-carboxylic acid in 5ml ammonia water (adjust pH to 8.0), add 1.5g (0.05mol) borane-ammonia complex, feed oxygen at a uniform speed, add 88.5 mg of recombinant D-amino acid oxidase and 18 mg of catalase were started to react at 28° C. under stirring, and the reaction progress was detected by HPLC. After about 28 hours, the HPLC test results showed that the ammonium salt of 1-(S)-tetrahydroisoquinoline-1-carboxylate was less than 1%. Stop the reaction, heat to 50-60°C, denature the enzyme protein for more than half an hour, filter the heated reaction through diatomaceous earth to remove the enzyme, add acetone twice the volume of the reaction solution to the filtrate to dilute, collect the precipitated crude solid by filtration, and then pass through Water / acetone (volume ratio 1 / 2) recrystallized to obtain 1.8 g ...
Embodiment 3
[0042] Preparation of embodiment 3 intermediate 1-(R)-tetrahydroisoquinoline formic acid potassium salt
[0043] 1.77g (0.01mol) DL-tetrahydroisoquinoline-1-carboxylic acid was dissolved in 5ml K 2 HPO 4 -KH 2 PO 4 In the buffer solution (adjust the pH to 8.2), add 2.61g (0.03mol) borane-tert-butylamine complex, feed oxygen at a uniform speed, add 35.5mg recombinant D-amino acid oxidase, 9mg catalase, stir The reaction was started at 35°C, and the reaction progress was detected by HPLC. After about 30 hours, the HPLC test results showed that the potassium salt of 1-(S)-tetrahydroisoquinoline-1-carboxylate was less than 1%. Stop the reaction, heat to 50-60°C, denature the enzyme protein for more than half an hour, filter the heated reaction through diatomaceous earth to remove the enzyme, extract the filtrate with toluene (3x5ml), and recover tert-butylamine (2.1g) from the toluene phase. Add 2 times the volume of acetone to the extracted water phase to dilute the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com