Particle composition as well as preparation method and preparation thereof
A technology for compositions and compound preparations, applied in the field of pharmaceutical preparations, to achieve the effects of improving stability, improving incompatibility difficulties, and overcoming incompatibility taboos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
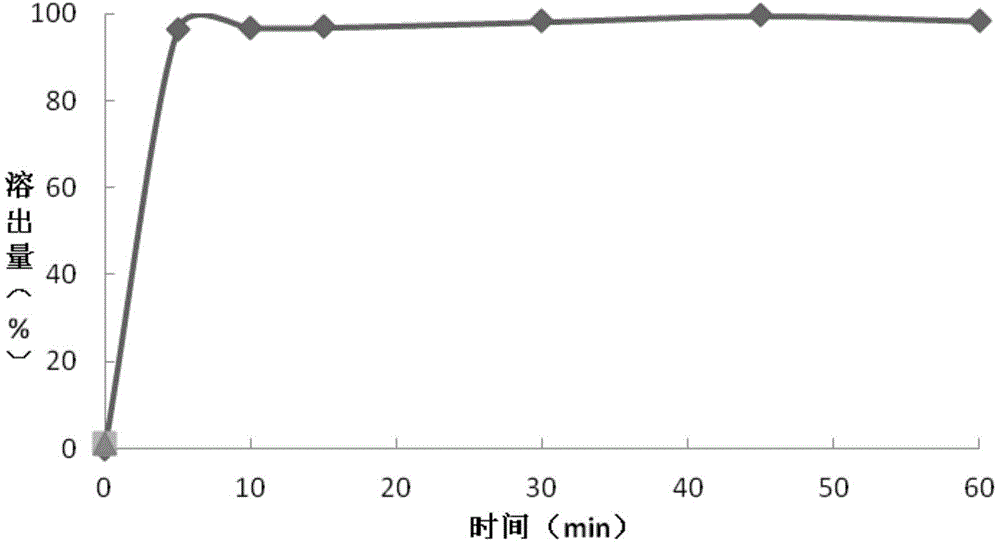
[0075] The preparation of embodiment 1 granular composition
[0076] The formulation of the granular composition is shown in Table 1.
[0077] Table 1 Prescription of Granular Composition
[0078]
[0079] Preparation of Montelukast sodium granules: Add purified water into a stainless steel bucket, add meglumine and stir until dissolved; continue stirring and add hydroxypropyl-β-cyclodextrin until dissolved, and finally add Montelukast sodium and stir until dissolved. Dissolve to obtain the first binder solution and set aside. Put the mannitol in a wet granulator after passing through a 26-mesh sieve, put the prepared first binder solution in a wet-process granulator, and make soft materials, which pass through a 26-mesh sieve for granulation, and dry at 45°C for 15 Minutes later, pass through a 26-mesh sieve for granulation, continue to dry until the loss on drying is ≤2.0% (IR90°C, dry for 10 minutes), pass through a 26-mesh sieve for granulation, and obtain montelukast...
Embodiment 2
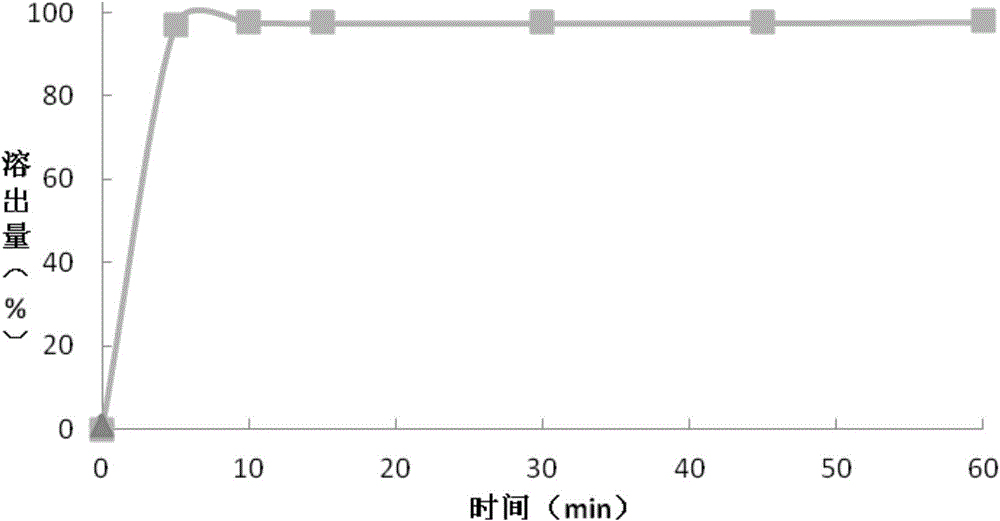
[0082] The preparation of embodiment 2 granular composition
[0083]The formulation of the granular composition is shown in Table 2.
[0084] Table 2 Prescription of Granular Composition
[0085]
[0086] Preparation of Montelukast sodium granules: Add purified water into a stainless steel bucket, add meglumine and stir until dissolved; continue stirring and add hydroxypropyl-β-cyclodextrin until dissolved, and finally add Montelukast sodium and stir until dissolved. Dissolved to obtain the first binder solution, set aside. Mannitol and microcrystalline cellulose 101 were respectively passed through a 26-mesh sieve, mixed evenly and placed in a wet granulator, and the prepared first binder solution was placed in a wet granulator to make soft materials, and the soft materials were passed through Granulate with a 26-mesh sieve, dry at 45°C for 15 minutes, pass through a 26-mesh sieve for granulation, continue to dry until the drying weight loss ≤ 2.0% (IR90°C, dry for 10 mi...
Embodiment 3
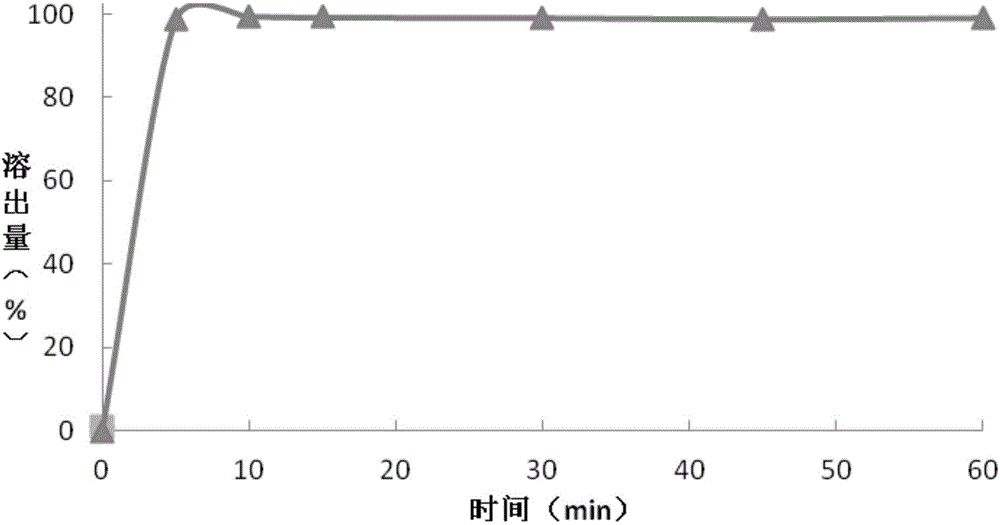
[0089] The preparation of embodiment 3 granular composition
[0090] The formulation of the granular composition is shown in Table 3.
[0091] Table 3 Prescription of Granular Composition
[0092]
[0093]
[0094] Preparation of Montelukast sodium granules: Add purified water into a stainless steel bucket, add meglumine and stir until dissolved; continue stirring and add hydroxypropyl-β-cyclodextrin until dissolved, and finally add Montelukast sodium and stir until dissolved. Dissolved to obtain the first binder solution, set aside. Put the mannitol in a wet granulator after passing through a 26-mesh sieve, put the prepared first binder solution in a wet-process granulator, and make soft materials, which pass through a 26-mesh sieve for granulation, and dry at 45°C for 15 Minutes later, pass through a 26-mesh sieve for granulation, continue to dry until the loss on drying is ≤2.0% (IR90°C, dry for 10 minutes), pass through a 26-mesh sieve for granulation, and obtain m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com