Porcine thrombin carbon terminal derived antibacterial peptide and preparation method and application thereof
A pig thrombin, antimicrobial peptide technology, applied in biochemical equipment and methods, antibacterial drugs, enzymes and other directions, can solve the problems of drug residues, environmental pollution, drug-resistant strains, etc., to achieve low synthesis cost, good safety, The effect of broad-spectrum antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Design of Antimicrobial Peptides Derived from Porcine Thrombin Carbon-Terminal
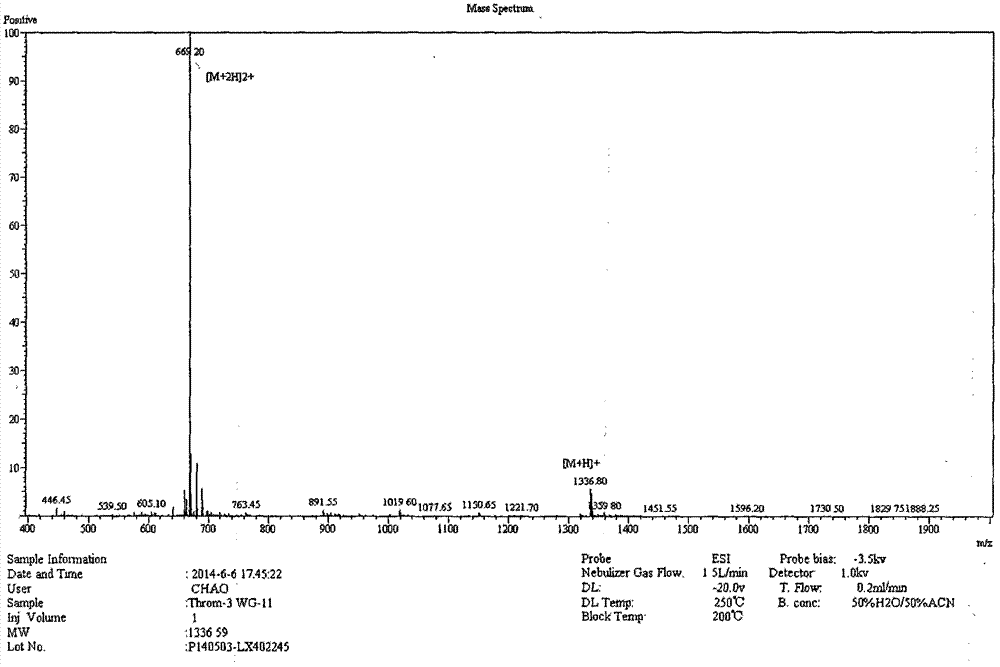
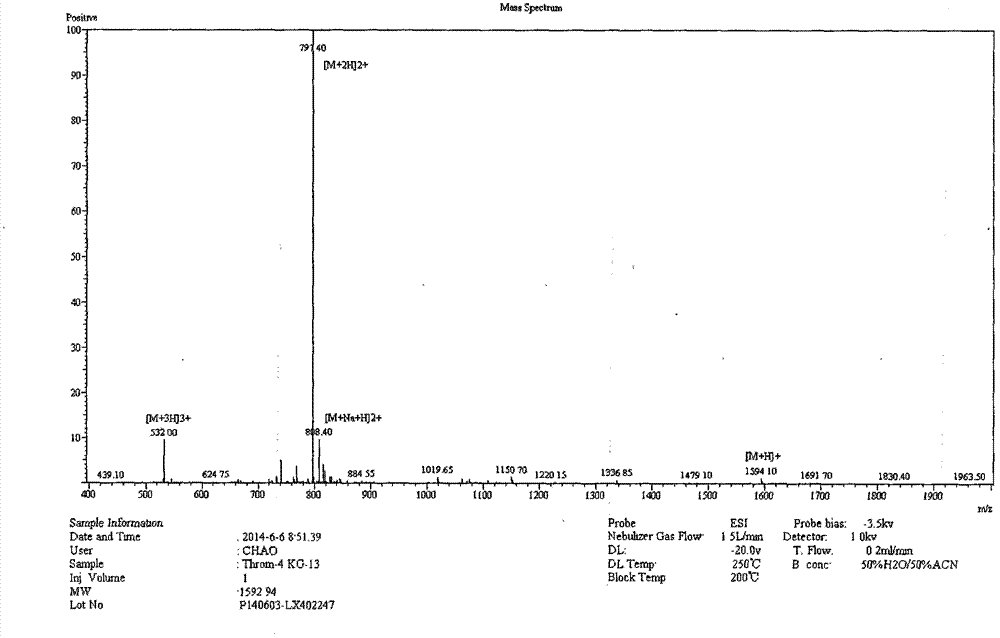
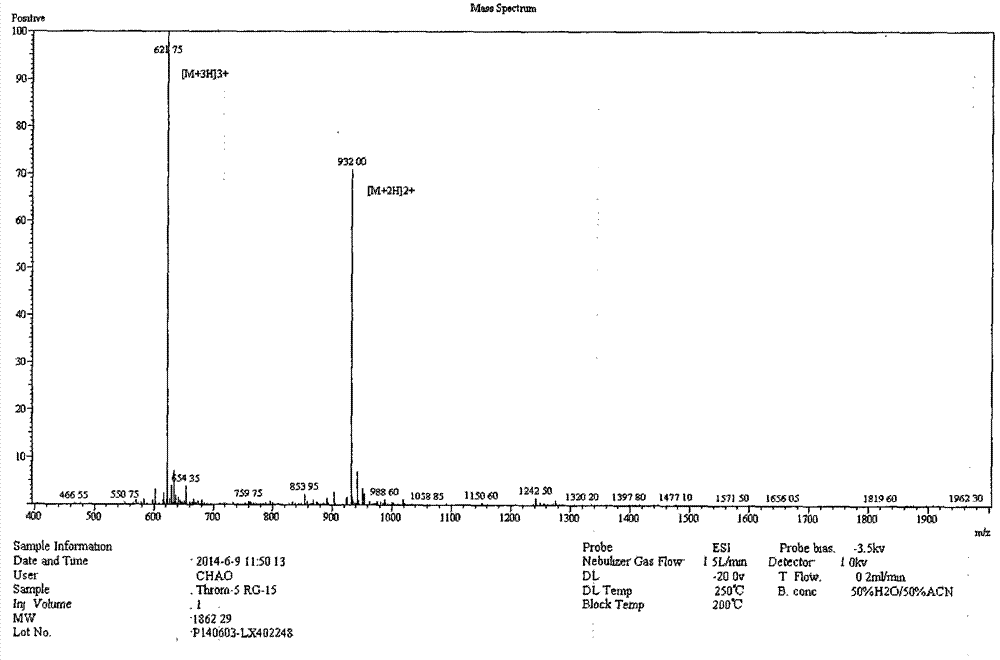
[0021] The complete amino acid sequence of porcine thrombin was obtained through NCBI, starting from the first amino acid at the carbon-terminal of the enzyme, and by truncation, five derived peptides ranging in length from 11 to 19 amino acids were obtained, PT11, PT13, PT15, PT17, PT19. As shown in Table 1.
[0022] Table 1 Amino acid sequences and molecular weights of antimicrobial peptides derived from porcine thrombin
[0023]
Embodiment 2
[0025] Synthesis of Antimicrobial Peptides Derived from Porcine Thrombin Carbon-Terminal
[0026] The five polypeptides shown in Table 1 were synthesized using a peptide synthesizer, using solid-phase organic synthesis, using the Fmoc protection synthesis method, and the synthesis direction was carried out one by one from the C-terminal to the N-terminal. The specific steps are as follows:
[0027] (1) Select the Wang resin that has been connected to the first amino acid at the C-terminal, that is, Fmoc-A(trt)-Wang (9-fluorenylmethoxy-trimethyl-A, where A is the first amino acid at the C-terminal) , use dimethylformamide (DMF) to soak for about 15 minutes to remove impurities; use DMF containing 20% piperidine to remove the Fmoc protection on the resin, react for 20 minutes, and wash the resin until it is complete. The piperidine was washed away with DMF, and the remaining solid suspension was the deprotected A-Wang. The quality of A-Wang deprotection was checked with ptrin...
Embodiment 3
[0032] Determination of Antibacterial Activity of Porcine Thrombin Carbon-terminal Derivatized Antibacterial Peptides
[0033] The minimum inhibitory concentration (MIC) method recommended by the American Clinical Laboratory Standardization Institute (CLSI) was adopted, and corresponding improvements were made for the cationic characteristics of antimicrobial peptides. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 50 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor antimicrobial peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhibitory concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com