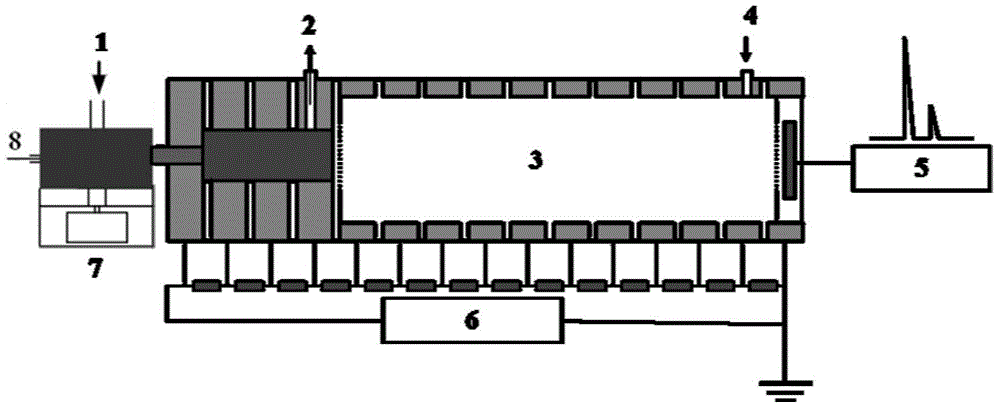
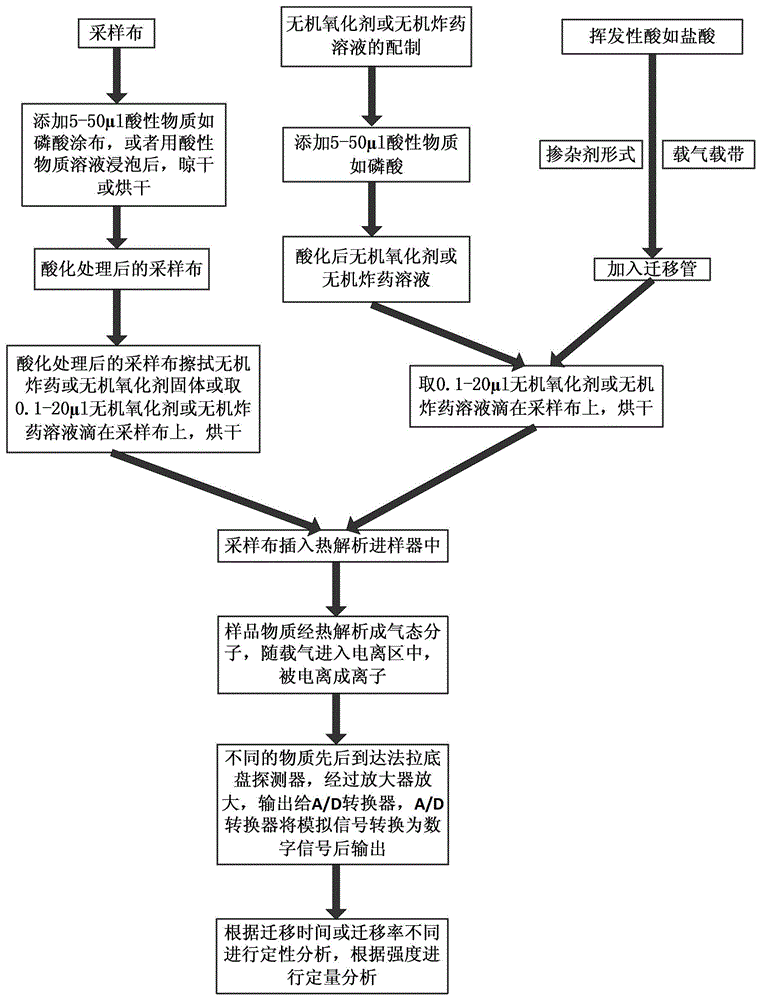
Method for measuring inorganic oxidants in inorganic explosive through thermal desorption ion mobility spectrometry
An inorganic oxidant and ion mobility spectrometry technology, applied in the field of inorganic oxidant, can solve the problems of analytical reproducibility and sensitivity deviation, unreliable qualitative conclusions, and difficult to use portablely, etc., to achieve convenient and rapid detection, fast measurement speed, and simple measurement method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
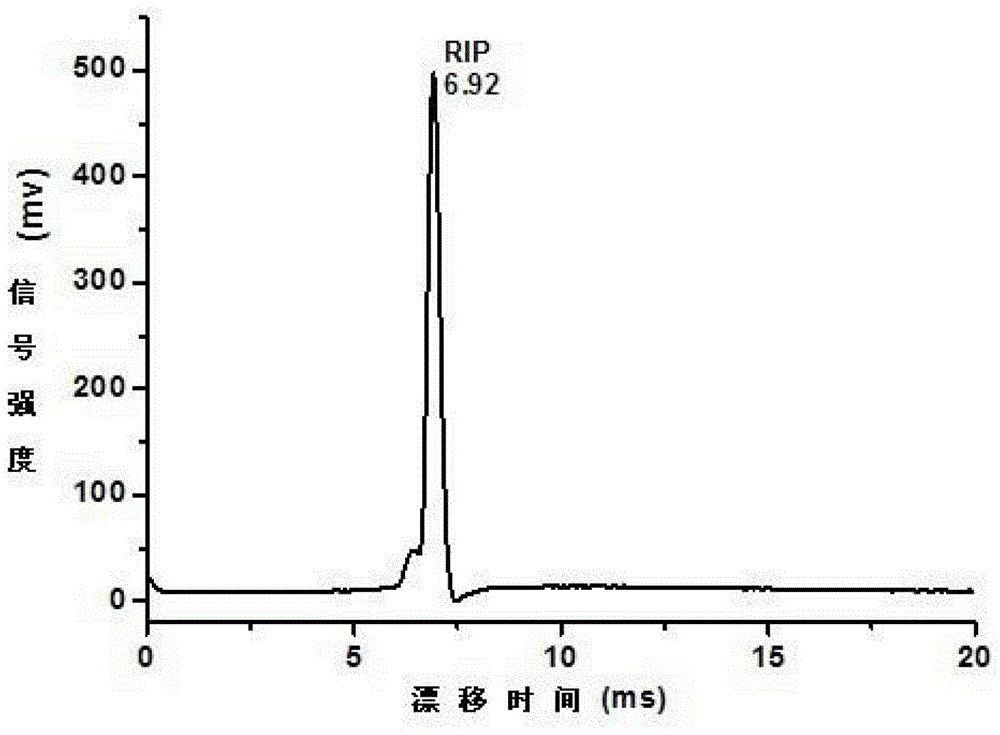
[0035] According to the above embodiment, 50 ng of potassium nitrate, potassium chlorate, potassium perchlorate and sodium nitrite were directly measured without acidification treatment. Figure 3-5 Reagent ion peak (RIP) in negative ion mode, background spectra of three acids used to acidify the sample cloth or inorganic oxidant solution, and ion mobility spectra of potassium nitrate, potassium chlorate, potassium perchlorate, and sodium nitrite without acidification picture. The migration time of the RIP peak in the negative ion mode is 6.29ms. Without any acidification treatment, almost no signal can be obtained in the IMS measurement of potassium nitrate, potassium chlorate, potassium perchlorate and sodium nitrite.
Embodiment 2
[0037] The four substances of potassium nitrate, potassium chlorate, potassium perchlorate and sodium nitrite after acidification treatment in two ways were measured according to the above-mentioned embodiment.
[0038] Image 6 It is the spectrum measured by ion mobility spectrometry after adding 20ul 3% phosphoric acid to 1ml of potassium nitrate, potassium chlorate, potassium perchlorate and sodium nitrite standard solution with a concentration of 50ng / μl; Figure 7 After the sample cloth was acidified with 5μl 3% phosphoric acid, ion mobility spectrometry was used to measure the spectra of the four standard solids of potassium nitrate, potassium chlorate, potassium perchlorate and sodium nitrite. The two figures show that in the presence of acidic substances, the four inorganic salts Whether solid or solution can be better measured.
Embodiment 3
[0040] The potassium nitrate standard solution after acidification treatment with different acids was measured according to the above-mentioned embodiment.
[0041] Figure 8 The spectrogram measured by ion mobility spectrometry after adding 20ul 3% phosphoric acid, boric acid and 1% acetic acid to 1ml 50ng / μl potassium nitrate solution for acidification shows that the acidification effect of phosphoric acid and boric acid is better, but the acidification effect of acetic acid is not very ideal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com