Recombinant TRAIL protein and preparation method and application thereof
A proteasome inhibitor and application technology, applied in the field of recombinant TRAIL protein and its preparation and application, can solve the problems of short half-life and instability, and achieve the effects of strong stability, improved use safety and long in vivo half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Cloning of embodiment 1mmTRAIL protein coding gene
[0057] 1) Extraction of rhesus monkey RNA
[0058] Extract venous blood from healthy rhesus monkeys, add it to a centrifuge tube filled with an appropriate amount of density gradient separation solution, and centrifuge at room temperature at 400 g for 30 min. Collect the peripheral blood mononuclear cells in a new centrifuge tube, then add 0.83% NH 4 Cl, let stand at room temperature for 5 minutes, and remove residual red blood cells. The cells were washed twice with phosphate buffer (50 mM, pH 7.4), suspended in 1640 medium containing 100 U / ml penicillin, 100 mg / ml streptomycin, and 10% fetal bovine serum to make the cell density 10 6 individual / ml. Add 5 μg / ml ConA to the cells to stimulate culture for 2 days. Collect cells to 10 7 Add 1-2ml RNAiso Plus Lysis Solution to each cell. After the cells are fully lysed, centrifuge at 12,000g at 4°C for 5min, and discard the pellet. Add 1 / 5 volume of chloroform of R...
Embodiment 2
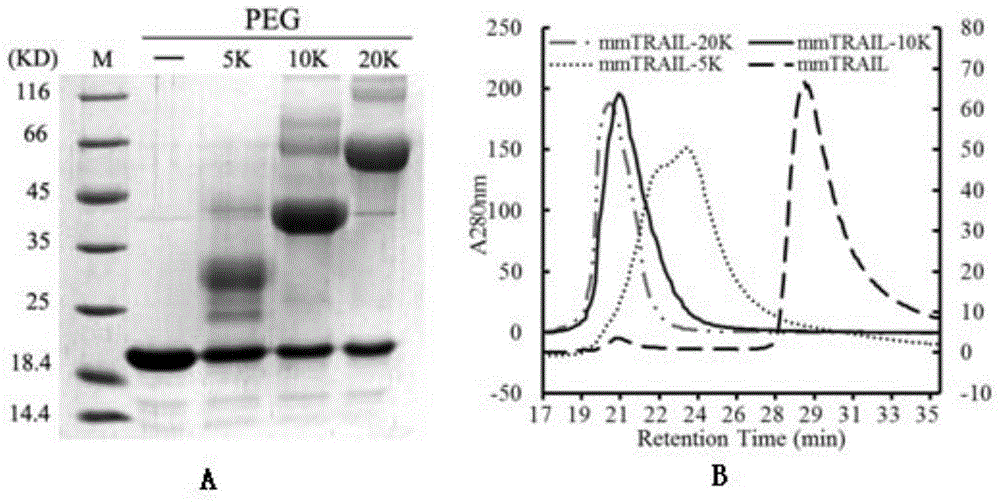
[0069] Recombinant expression and separation and purification of embodiment 2mmTRAIL protein
[0070] 1. Preparation method
[0071] Extract the pQE30-mmTRAIL plasmid prepared in Example 1, add it to Escherichia coli M15 competent cells, and transform it with the heat shock method. Solid plates were screened. Pick positive clones and inoculate them in 5ml LB liquid medium (containing 100 μg / mL ampicillin and 30 μg / ml kanamycin), and culture overnight at 37°C with shaking. The overnight culture bacteria were inoculated into fresh LB culture medium at a ratio of 1:1000, and continued to culture until bacterial solution A 600 When it reaches about 0.5, add 0.1mM IPTG, 200μM ZnSO 4 Induce overnight at 28°C.
[0072] Collect the cells by centrifugation at 7000g for 10min, resuspend the cells obtained per liter of fermentation broth with 20-30ml lysate (50mM phosphate buffer, pH8.0, 300mM NaCl, 20mM imidazole), then add 1mM PMSF, 10mM β-mercaptoethanol . Ultrasonic bacteria de...
Embodiment 3
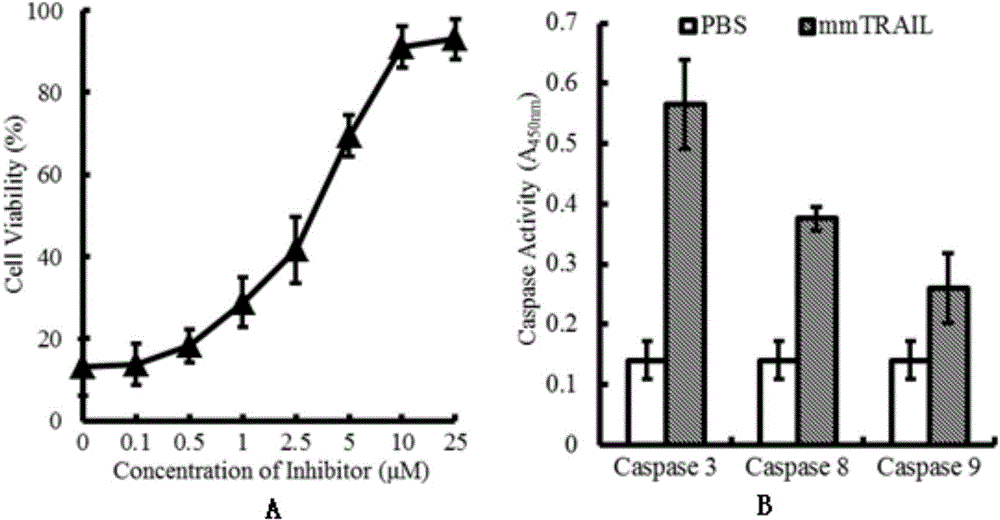
[0080] Example 3 The killing effect of mmTRAIL protein on tumor cells in vitro
[0081] Tumor cell lines:
[0082] Colo205 cells: colon cancer cells; U87 cells: brain astroglioblastoma cells; Jurkat cells: peripheral blood leukemia T cells; SMMC-7721 cells: liver cancer cells; M14: melanoma cells; SW620 cells: colon cancer cells; PLC cells: liver cancer cells; A549: lung adenocarcinoma cells; HEP3B cells: liver cancer cells.
[0083] 1) The killing effect of mmTRAIL alone on sensitive tumor cells
[0084] experimental method:
[0085] Cells were cultured in RPMI1640 containing 10% calf serum, 2 mM L-glutamine, 100 μg / ml streptomycin and 100 U / ml penicillin at 37 °C, 5% CO 2 cultivated under conditions. Will 1×10 4Cells (100 μl) were inoculated in a 96-well plate and adhered overnight, and then the medium was replaced with 1640 medium containing 2% calf serum, and different concentrations (0, 0.1, 0.25, 0.5, 1, 2.5, 5 , 10 and 25nM) the mmTRAIL protein obtained in Example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com