Multi-fluorogenic quantitative PCR (polymerase chain reaction) method for detecting duck hepatitis A virus (DHAV) and identifying DHAV-A and DHAV-C and kit
A technology of multiple fluorescence quantification and duck hepatitis A, which is applied in the direction of microorganism-based methods, biochemical equipment and methods, and microorganism measurement/inspection, can solve the problems of difficult differential diagnosis and rare differential detection methods, and achieve Improve detection time and efficiency, high accuracy, and avoid false positive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Establishment of multiple fluorescent quantitative PCR method for detection of duck hepatitis A virus and identification of genotype A and C
[0051] (1) Primer probe design
[0052] Download all currently known DHAV genome sequences of different genotypes from the GenBank database, use BioEdit software for homology sorting analysis and comparison, and determine the DHAV3D region as the design region for detecting DHAV universal primers and probes; select DHAV-A and DHAV respectively The conserved region in the 5'UTR sequence of DHAV-C is used as the design region for DHAV-A and DHAV-C detection primers and probes. Finally, the Beacon Designer7.0 biological software is used to design multiple groups that meet the requirements of fluorescent quantitative PCR for the above regions. For the primers and probes, the specificity of the primers and probes was firstly analyzed by BLAST theory, and then the best combination of primers and probes was further screened ou...
Embodiment 2
[0095] Embodiment 2 detects genotype A duck hepatitis A virus infection
[0096] (1) Test sample
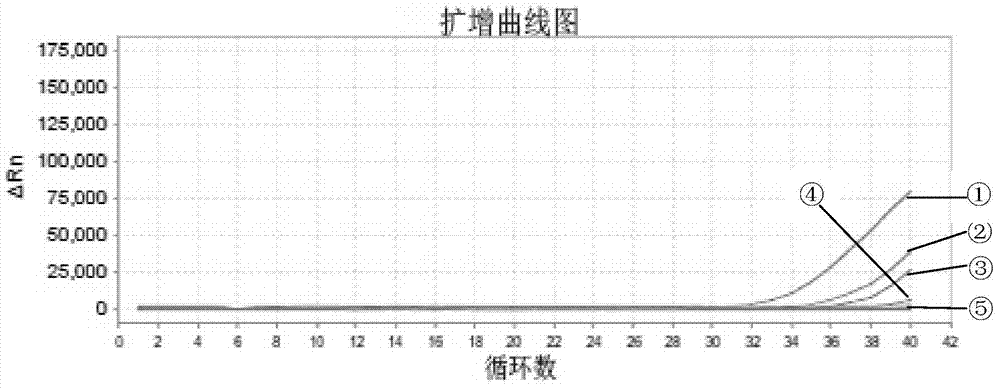
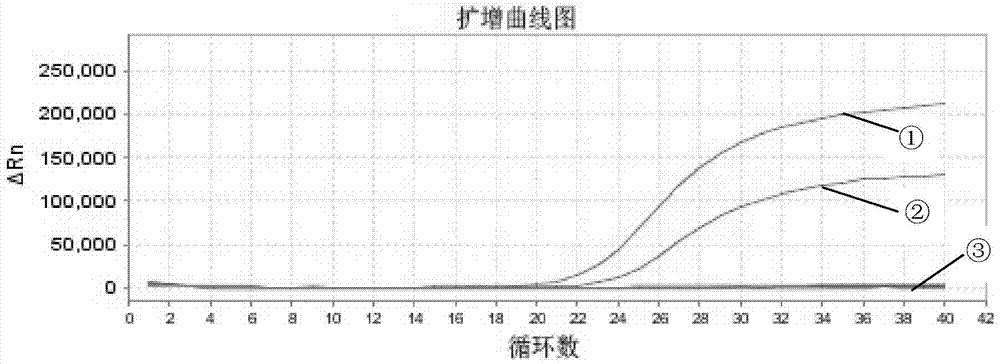
[0097] The selected detection samples are dead duck liver tissue (S1) infected with type A duck hepatitis A virus, healthy duck liver tissue (S2), chicken embryo tissue poisoned by type A duck hepatitis A virus (S3), healthy chicken embryo tissue (S4), A Duck hepatitis A virus chicken embryo attenuated allantoic fluid virus (S5), positive standard (P).
[0098] (2) Extraction of viral RNA
[0099] The extraction method of viral RNA refers to the above method, and the liquid nitrogen method is used to grind the tissue virus to prevent the heat generated during the grinding process from degrading the viral RNA. The nucleic acid extraction method of the tissue homogenate after grinding is the same as that of chicken embryo allantoic fluid virus, and the RNA is extracted by the traditional Trizol method.
[0100] (3) Reverse transcription and multiplex fluorescent quantitative PCR...
Embodiment 3
[0107] Embodiment 3 detects genotype C duck hepatitis A virus infection
[0108] (1) Test sample
[0109] The selected detection samples are dead duck liver tissue (Y1) infected by type C duck hepatitis A virus, healthy duck liver tissue (S2), duck embryo tissue poisoned by type C duck hepatitis A virus (Y2), healthy duck embryo tissue (D4), C Type Duck Hepatitis A Virus Duck Embryo Attenuated Allantoic Fluid Virus (Y3), Positive Standard (P).
[0110] (2) Extraction of viral RNA
[0111] The extraction method of viral RNA refers to the above method, and the liquid nitrogen method is used to grind the tissue virus to prevent the heat generated during the grinding process from degrading the viral RNA. The nucleic acid extraction method of the tissue homogenate after grinding is the same as that of chicken embryo allantoic fluid virus, and the RNA is extracted by the traditional Trizol method.
[0112] (3) Reverse transcription and multiplex fluorescent quantitative PCR detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com