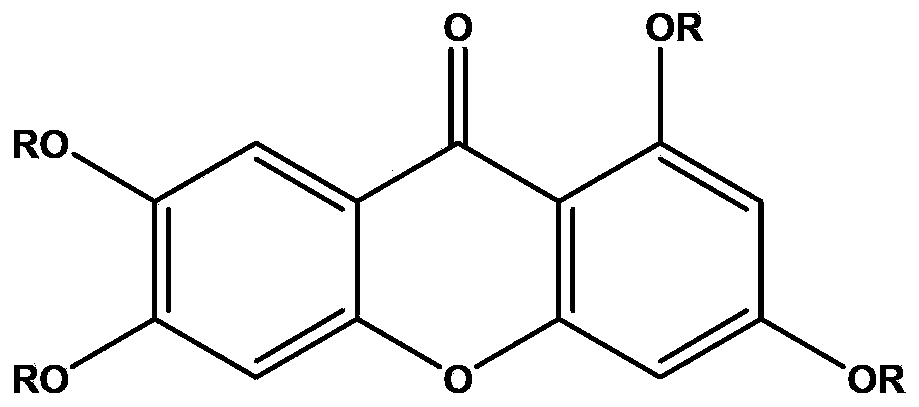
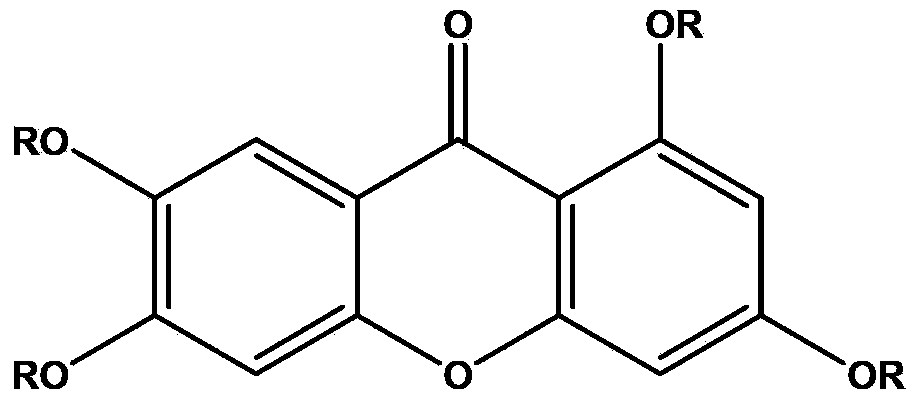
Application of 1,3,6,7-tetrahydroxy diphenylpyrrone derivative in preparing drug for preventing hyperuricemia and/or gout
A kind of technology of tetrahydroxybenzopyrone and its derivatives, applied in the field of medicine, can solve the problems affecting the quality of life, abnormality, diarrhea, headache, joint-related signs and symptoms and musculoskeletal/connective tissue symptoms, see gastrointestinal reactions, Rashes, fever and other adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 The research on the uric acid excretion effect of 1,3,6,7-tetrahydroxybenzopyrrole derivatives shown in formula I
[0069] Select 21 male Wistar rats, weighing 340±20g, provided by the Experimental Animal Center of Kunming Medical University [Experimental animal production license number: SCXK (Dian) 2005-2008]. Breeding conditions: Room temperature is 22±2°C, relative humidity is 60% to 70%.
[0070] The isolated rat kidney perfusion model was prepared according to the method reported by Wang and Lepsy, and the perfusion fluid was K-H solution (mmol / L, NaCl118.0, KCl4.7, NaHCO 3 25,KH 2 PO 4 1.2, MgSO 4 2.34, CaCl 2 1.28, glucose5.55) as the basis, plus 65g / L bovine serum albumin component V and 0.153% compound amino acid injection (17AA, added before use). After preparation, filter with a membrane filter (0.45 μm pore size), and adjust the pH to 7.4. 1h before use and during the perfusion process, continue to fill with 95% O 2 +5%CO 2 mixed compositi...
Embodiment 2
[0083] Influence of 1,3,6,7-tetrahydroxybenzophenone derivatives shown in embodiment 2 formula I on xanthine oxidase
[0084] Xanthine oxidase (EC1.1.3.22) was prepared with PBS to an appropriate concentration for later use. Tetraacetoxybifen was prepared in PBS to a series of concentrations (1.2mmol / L, 0.12mmol / L, 0.012mmol / L) for later use.
[0085] After incubating the tetraacetoxydiphenhydrazine solution with serial concentrations and xanthine oxidase at 37°C for 30 minutes, the activity of xanthine oxidase was measured with a xanthine oxidase assay kit to obtain a series of concentrations of tetraacetoxydiphenylpyrrolidone The inhibition rate of piroxin solution to xanthine oxidase is shown in Table 2.
[0086] The impact of table 2 tetraacetoxy benzophenone on xanthine oxidase ( n=9)
[0087]
[0088] It can be seen from Table 2 that the inhibitory rates of tetraacetoxybenzophenone at serial concentrations (1.2mmol / L, 0.12mmol / L, 0.012mmol / L) on xanthine oxidase a...
Embodiment 3
[0094] Example 3 Study on the Dose-Effect Relationship of 1,3,6,7-tetrahydroxybenzopyrrole Derivatives in Lowering Uric Acid Shown in Formula I
[0095] Select 60 healthy male Kunming mice, weighing 18-22 g, provided by the Experimental Animal Center of Kunming Medical University [Experimental animal production license number: SCXK (Dian) 2005-2008]. Breeding conditions: Room temperature is 22±2°C, relative humidity is 60% to 70%.
[0096] Animals were randomly divided into 6 groups, 10 in each group, respectively normal control group (0.5%CMC-Na10ml / kg), hyperuricemia model group (oxonate potassium 250mg / kg), tetraacetoxydiphenylpyrrole Ketone low-dose group (potassium oxonate 250mg / kg+tetraacetoxybifen 1.52mg / kg), medium-dose group tetraacetoxybifen (potassium oxonate 250mg / kg+tetraacetoxy phenpyrazone 3.11mg / kg), tetraacetoxy benzopyrone high-dose group (oxonate potassium 250mg / kg + tetraacetoxy benzopyrone 6.47mg / kg) and positive drug control group (oxonate potassium Pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com