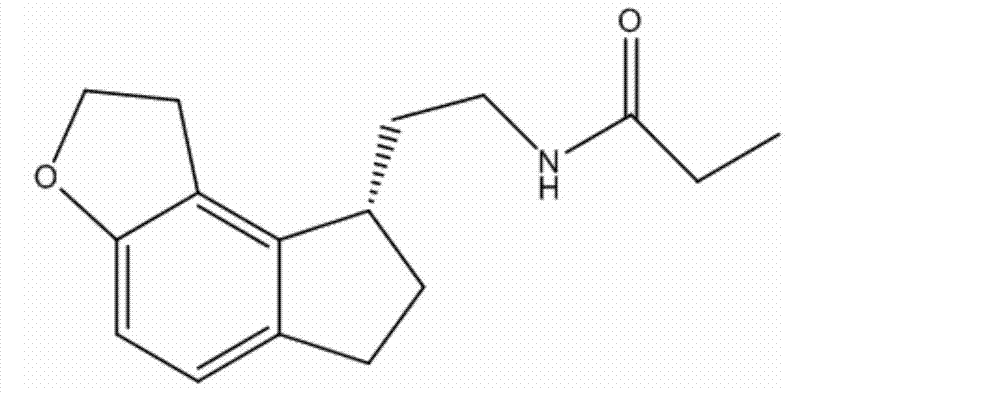
Ramelteon composition and tablet thereof
A technology of ramelteon tablets and ramelteon, which is applied in the field of medicine, can solve the problems of unstable chemical properties of ramelteon, poor stability of ramelteon tablets, and difficulty in industrial production, and meet equipment requirements Not high, fast dissolution rate, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] According to the present invention, the preparation method of the ramelteon tablet is preferably wet granulation compression, dry granulation compression or direct powder mixing compression; more preferably wet granulation compression. The wet granulation tablet preferably comprises the following steps:
[0043] Carry out micronization treatment of ramelteon, then mix the micronized ramelteon with filler and disintegrant evenly, then add the ethanol solution of copovidone to prepare soft material, then granulate, dry, The granules are sized, and then a lubricant is added to mix evenly, compressed into tablets, and finally coated to obtain ramelteon tablets.
[0044] According to the present invention, the micronization treatment controls the particle size of ramelteon to D90≤40 μm, which can ensure the rapid dissolution of the drug, and has low requirements on the equipment of the pulverizer.
[0045] The ethanol solution is an aqueous ethanol solution, and the volume ...
Embodiment 1
[0051] Micronize ramelteon to control its particle size to D90≤40 μm, then mix 8 g of ramelteon micronized with 110 g of lactose and 10 g of starch, and then add 30% ethanol with 5 wt% copovidone Solution 13g, prepare soft material, then granulate at 24 mesh, ventilate and dry at 70°C, granulate at 24 mesh, then add 1g of magnesium stearate and mix evenly, press into tablets with a punch with a diameter of 7mm, and finally coat to obtain Lemei For the amine tablet; the weight gain of the coating is 4g, wherein the coating powder formula is composed as follows: 100 parts by weight of hydroxypropyl methylcellulose, 40 parts by weight of titanium dioxide, and 10 parts by weight of iron oxide yellow.
Embodiment 2
[0053] Micronize ramelteon to control its particle size to D90≤40 μm, then mix 8 g of ramelteon micronized with 110 g of lactose and 10 g of starch, and then add 30% ethanol with 10 wt% copovidone 13g of the solution, wherein 0.02g of vitamin E is dispersed in the ethanol solution, and the soft material is prepared, then granulated at 24 mesh, ventilated and dried at 70°C, and granulated at 24 mesh, and then 1g of magnesium stearate is added to mix evenly, and a punch with a diameter of 7mm is used. Head compression tablet, carry out coating at last, obtain ramelteon tablet; Described coating increases in weight 4g, and wherein, coating powder formula is composed as follows: 100 parts by weight of hydroxypropyl methylcellulose, 40 parts by weight of titanium dioxide , 10 parts by weight of iron oxide yellow.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com