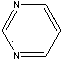
N-gallic amide-pyrimidine phenyl sulfonamide derivative as well as preparation method and application thereof
A technology of pyrimidinylbenzenesulfonamide and sulfadiazine is applied in the field of medicine and preparation, and can solve the problems of weak pharmacological activity, low drug transmembrane permeability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 3,4,5-Triacetylgalloylsulfadiazine
[0035] In an oil bath at 80°C, mix gallic acid with 3 times (m:V) acetic anhydride and stir for 12 hours, add 15 times the volume of distilled water and let it stand for 48 hours, filter the insoluble matter, and dry it in vacuum to obtain 3,4,5- Triacetyl gallic acid; reflux 3,4,5-triacetyl gallic acid and thionyl chloride in an oil bath at 70°C for 6 hours, and recover thionyl chloride under reduced pressure to obtain 3,4,5-triacetyl base galloyl chloride; mix 3,4,5-triacetyl galloyl chloride and sulfadiazine sodium in tetrahydrofuran, add a small amount of pyridine as a catalyst, stir in ice bath for 2 hours and room temperature for 12 hours, then add distilled water, reduce After part of the THF was evaporated under pressure, it was allowed to stand for 48 hours, and the insoluble matter was collected by filtration, dried in vacuo, and recrystallized in a methanol-THF system to obtain 3,4,5-triacetylgalloylsulfadiazine.
Embodiment 2-4
[0037] With the operation of Example 1, replace the sodium sulfadiazine in Example 1 with sulfa-methoxine, sulfa-p-methoxine, and sulfamethoxine respectively, and the rest of the operations are the same as in Example 1 to obtain 3,4,5-triacetyl Galloyl sulfa-methoxine, 3,4,5-triacetyl sulfa-methoxine, 3,4,5-triacetyl sulfa-methoxine.
Embodiment 5
[0039] 3,4,5-Trihydroxygallosulfamethazine
[0040] The same operation as in Example 1, using sulfamethazine instead of sulfamethazine in Example 1, after obtaining 3,4,5-triacetyl sulfamethazine, dissolve it in tetrahydrofuran, add hydrochloric acid at 60°C for hydrolysis After 6 hours, add distilled water, evaporate part of tetrahydrofuran under reduced pressure at 80°C, let it stand, filter the insoluble matter, dry it in vacuum, and recrystallize in methanol-tetrahydrofuran system to obtain 3,4,5-trihydroxygallosulfamethazine .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com