HIV-1 multi-epitope recombinant protein, and encoding gene and application thereof
A HIV-1 coding technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problem of no vaccine application, etc., and achieve good safety, strong immunogenicity, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
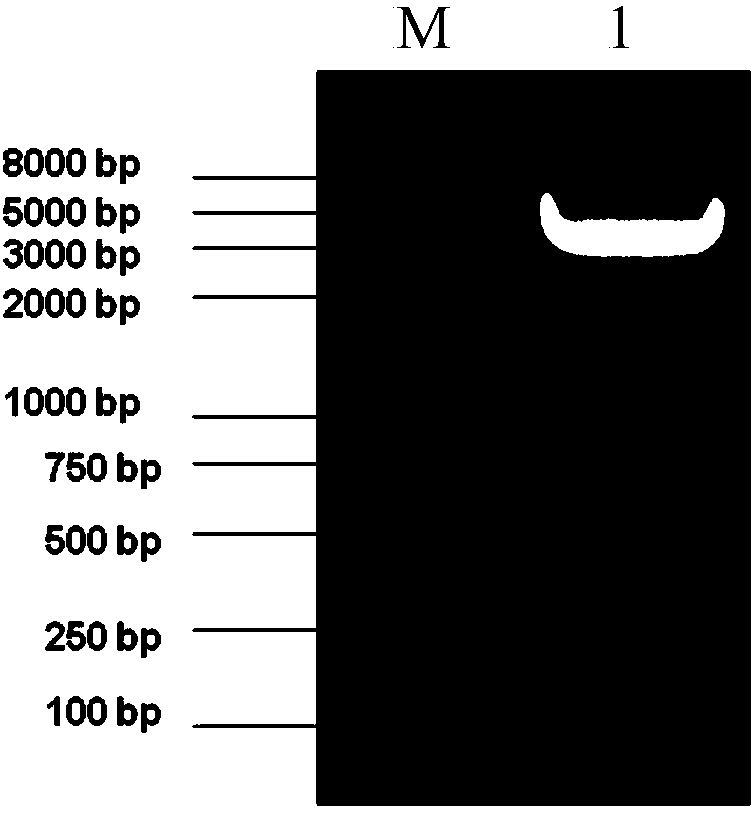
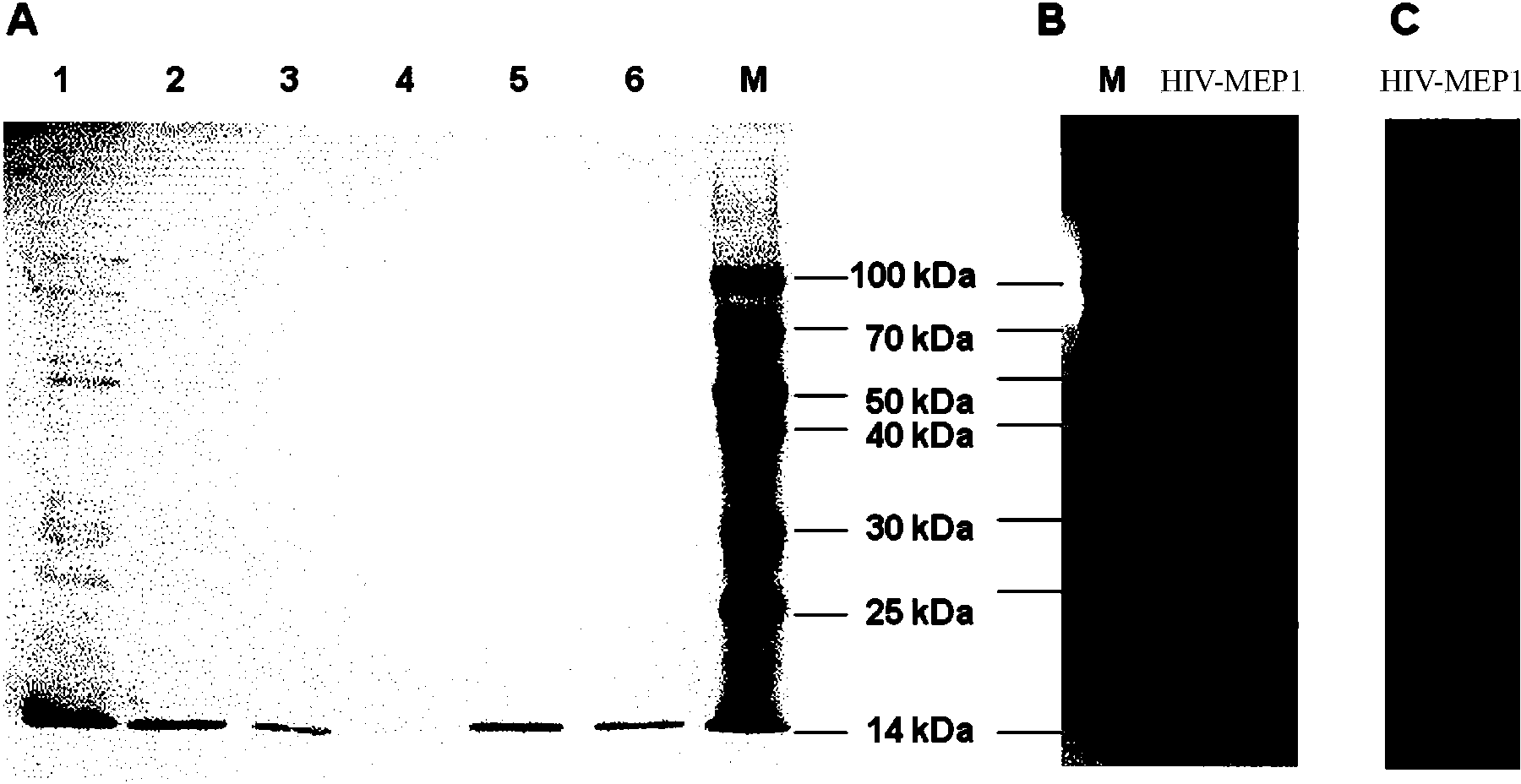
[0053] Example 1, Expression and purification of HIV-1 multi-epitope recombinant protein HIV-MEP1
[0054] 1. Conserved epitope screening and DNA sequence synthesis in line with the restriction of dominant MHC in the Chinese population
[0055] Obtain all reported epitopes on the env, gag and pol genes of the main HIV-1 epidemic strains in China (CRF01_AE, CRF07_BC, CRF08_BC, B) from HIV Databases, and screen out all epitopes that are conserved in sequence and conform to the MHC-restricted dominant Chinese population , the epitopes screened were further connected in series with the linker sequence GGGS and codon-optimized in Escherichia coli, and the corresponding double-stranded DNA was synthesized in Nanjing GenScript Company (and BamH I and Hind III recognition sequences were added at both ends), as shown in No. 31-499 of Sequence 2 in the Sequence Listing (No. 31-36 is the BamH I recognition sequence, No. 494-499 is the Hind III recognition sequence, and No. 493-495 is the...
Embodiment 2
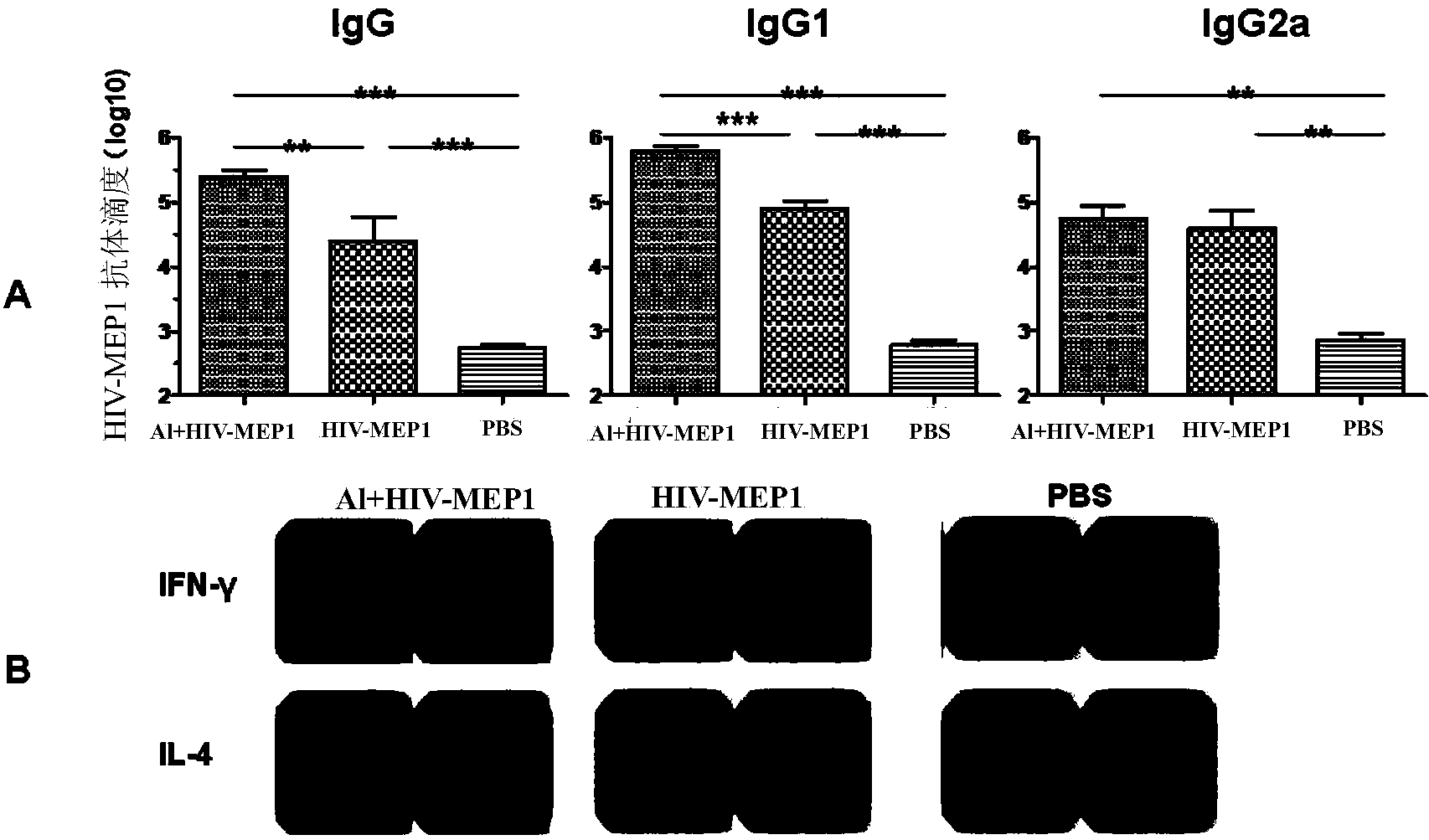
[0067]Example 2, Immunological evaluation of HIV-1 multi-epitope recombinant protein HIV-MEP1 in BALB / c mice
[0068] 1. Animal immunization and specimen collection:
[0069] Female BALB / c mice (6-8 weeks old) were randomly divided into three groups, i.e. recombinant protein aluminum adjuvant group (group 1), recombinant protein alone immunization group (group 2) and PBS control group (group 3), A total of three immunizations were performed, with the time interval of each immunization being 4 weeks, and the dose of each immunization was the same. The grouping situation and immunization strategy are shown in Table 1.
[0070] Table 1 Animal immunization experiment grouping and immunization strategy
[0071] group
quantity
Immunoreagent
immune dose
way of immunization
1
6
Aluminum Adjuvant+HIV-MEP1 / PBS
10μg / 200μl
2
6
HIV-MEP1 / PBS
10μg / 200μl
3
5
...
Embodiment 3
[0101] Example 3. Immunological evaluation of HIV-1 multi-epitope recombinant protein HIV-MEP1 in human HLA A2.1 / DR1 transgenic mice
[0102] The experimental scheme of this embodiment is the same as that of Example 2, except that human HLA A2.1 / DR1 transgenic mice are used to evaluate cellular immunity (IFNγ), since human HLA A2.1 / DR1 transgenic mice are C57 / B6 Background transgenic mice, in order to evaluate whether the cellular immunity induced by HIV-1 multi-epitope recombinant protein HIV-MEP1 is restricted by human MHC, at the same time, C57 / B6 mice were used as control for comparative study. The inoculation route, inoculation dose, and evaluation method are the same as in Example 2.
[0103] The results show:
[0104] (1) Stimulation results of HIV-1 multi-epitope recombinant protein HIV-MEP1 and 12 mixed polypeptides
[0105] Spleen cells of C57 / B6 mice were immunized with HIV-1 multi-epitope recombinant protein HIV-MEP1 and mixed polypeptides to stimulate aluminum a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com