Chiral core-shell chromatography stationary phase and preparation method
A core-shell type, core-shell technology, applied in the field of chiral core-shell liquid chromatography filler and its preparation, can solve the problems of small specific surface area, low content of chiral selector, etc., and achieve high permeability and fast analysis speed , not easy to lose the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Under nitrogen protection, in a 250mL two-necked flask, add 0.38g trans-(1R,2R)-cyclohexanediamine (DACH), 100mL anhydrous CH 2 Cl 2 , slowly drop 1.73g isocyanate propyltriethoxysilane (ICPTES) into the above reaction solution with a syringe, react at room temperature for 2h, and distill CH under reduced pressure 2 Cl 2 , to obtain a solid product. Then the resulting solid was washed with anhydrous n-hexane, filtered, and the excess ICPTES was removed together with the filtrate, and the filter cake was vacuum-dried to obtain chiral cyclohexanediamine disiloxane: N, N'-di-[(triethoxy Silyl)propyl]-trans-(1R,2R)-bis-(ureide)-cyclohexane (DACH-BS) as a white solid.
[0021] (2) Mix ammonia water, ethanol and deionized water at a volume ratio of 200:56:80 to make liquid A, add 2ml liquid A + 1ml tetraethyl orthosilicate (TEOS) to a 50ml round-bottomed flask, and place in an ice-water bath Stir for 5 minutes, and add liquid A and TEOS dropwise at room temperature wi...
Embodiment 2
[0025] (1) Under nitrogen protection, in a 250mL two-necked flask, add 0.38g trans-(1R,2R)-cyclohexanediamine (DACH), 100mL anhydrous CH 2 Cl 2 , slowly drop 1.73g isocyanate propyltriethoxysilane (ICPTES) into the above reaction solution with a syringe, react at room temperature for 2h, and distill CH under reduced pressure 2 Cl 2 , to obtain a solid product. Then the resulting solid was washed with anhydrous n-hexane, filtered, and the excess ICPTES was removed together with the filtrate, and the filter cake was vacuum-dried to obtain chiral cyclohexanediamine disiloxane: N, N'-di-[(triethoxy Silyl)propyl]-(1R,2R)-bis-(ureide)-cyclohexane (DACH-BS) as a white solid.
[0026] (2) Mix ammonia water, ethanol and deionized water at a volume ratio of 200:60:100 to prepare liquid A, mix and stir liquid A with tetraethyl orthosilicate at a volume ratio of 2:1 in an ice bath for 1.5 h, The obtained product was washed with deionized water and ethanol until neutral, and dried at 8...
Embodiment 3
[0030] figure 1 It is the scanning electron micrograph of the chiral cyclohexanediamine core-shell chromatographic filler in Example 1
[0031] figure 2 It is the transmission electron microscope figure of the chiral cyclohexanediamine core-shell chromatographic filler in Example 1
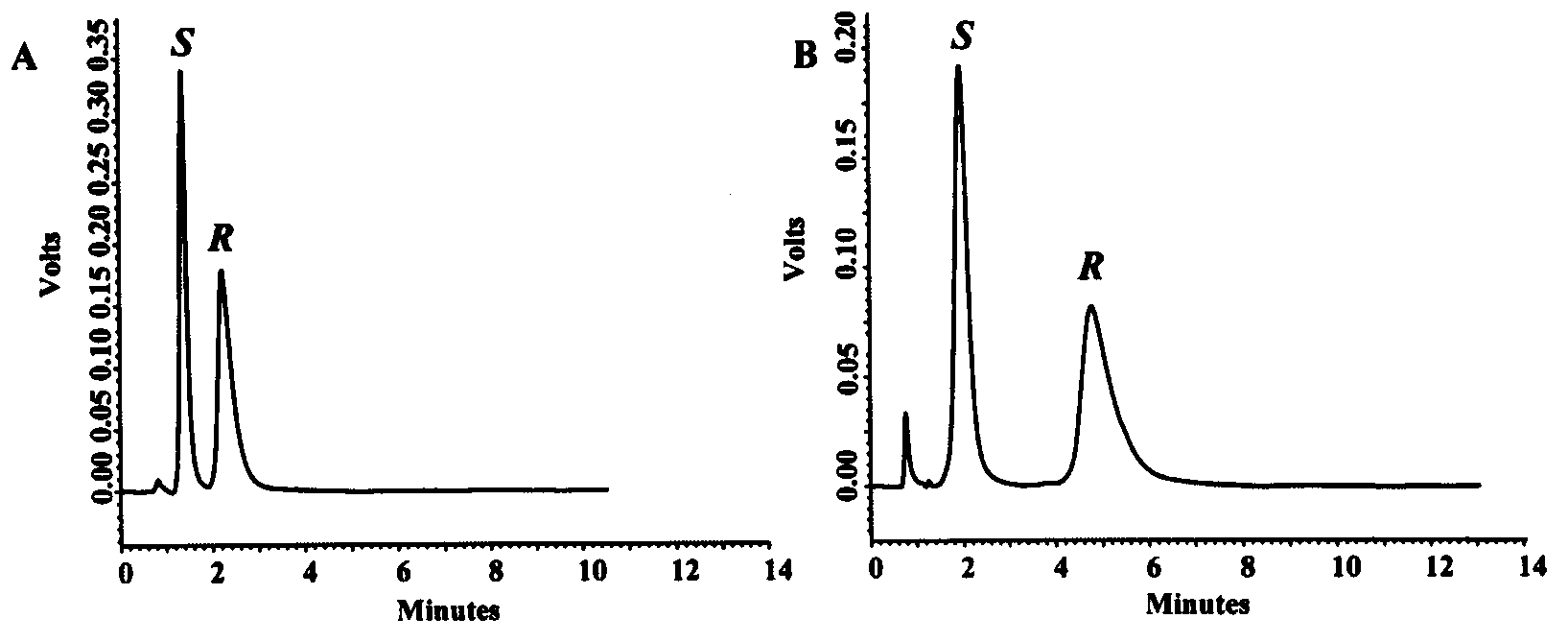
[0032] image 3 It is the chiral cyclohexanediamine core-shell chromatographic packing material of the present invention to separate (A) R / S-1,1'-binaphthalene-2,2'-diphenol, (B)R / S under normal phase system - Chromatogram of 6,6'-dibromo-1,1'-binaphthyl-2,2'-diol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com