Expression vector of Hainan toxin-iv analog rhniv-01 and preparation method thereof
An expression vector and technology of Hainan toxin, which is applied to the expression vector of Hainan toxin-IV analog rHNIV-01 and the field of preparation thereof, can solve the problems of high technical threshold and low degree of product homogeneity, and achieve the effect of a low-cost purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction and use of expression vector pWE-rHNIV-01
[0026] Using the pET-43a vector retained in our laboratory as a template, using the PCR-based seamless cloning method (for specific experimental methods, see Journal of Biochemical and Biophysical Methods67(1), 67–74.(2006)), the coding The sequence of GST (SEQ ID NO.1) replaced the original NusA sequence, and at the same time, the sequence encoding SUMO (SEQ ID NO.2) was inserted downstream of GST, and finally the sequence encoding rHNIV-01 (SEQ ID NO.3) was inserted Downstream of the ULP1 enzyme cleavage recognition site of SUMO ( figure 1 ). The PCR product was digested with DpnI and then transferred into E. coli DH5α. The plasmid was extracted and verified by DNA sequencing. Finally, the prokaryotic expression plasmid containing the expression cassette of GST+SUMO+rHNIV-01 was obtained and named pWE-rHNIV-01. The sequence is as shown in SEQ Shown in ID NO.4.
[0027] The primers used are as follow...
Embodiment 2
[0043] Example 2 Induced expression of expression plasmid pWE-rHNIV-01
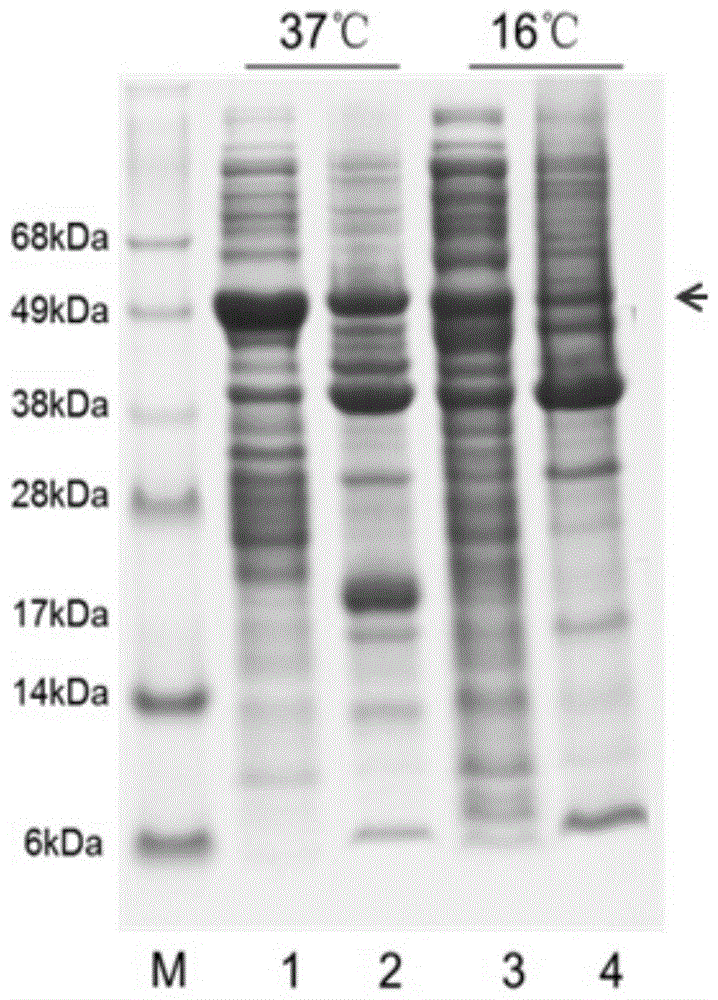
[0044] Using the conventional calcium chloride transformation method, the expression plasmid was transformed into Escherichia coli BL21(DE3), and then spread on 1 ampicillin-resistant (100 μg / mL) LB solid medium, and placed upside down at 37 degrees for 16 hours. The next day, pick 1-2 single colonies and inoculate them in 5 mL of ampicillin-resistant LB culture medium, and cultivate them at 37 degrees and 220 rpm until OD 600 To around 1 (this step is the enrichment of the seed medium). Transfer 5mL seed culture medium into fresh 500mL LB (100μg / mL ampicillin) culture solution, and continue to cultivate at 37°C and 220rpm until OD 600 to around 0.6, add IPTG with a final concentration of 0.5mM at this time to induce expression at 37 degrees, and the induction time is 4 hours. Through experiments, we found that the fusion protein containing rHNIV-01 can also be expressed normally in a partially soluble ...
Embodiment 3
[0045] Example 3 Preliminary separation and purification of rHNIV-01 fusion protein and protein kinase digestion
[0046] The thallus that has been induced in Example 2 was centrifuged at 4500 g for 10 minutes to obtain the thallus. Then add an appropriate volume of PBS buffer to resuspend the cells, followed by sonication. After the sonication is completed, centrifuge at 15,000 rpm for 10 minutes to obtain supernatant and precipitate. A dialysis membrane of appropriate length (molecular weight cut-off: 35 kDa) was used to dialyze the ultrasonic supernatant after centrifugation, and dialyze overnight at 4°C. After the dialysis is completed, the liquid in the dialysis bag is subjected to high-speed centrifugation, and the dialysis supernatant is taken for subsequent experiments. An appropriate dose of ULP1 kinase (to release rHNIV-01) was added to the supernatant and digested at 30°C for 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com