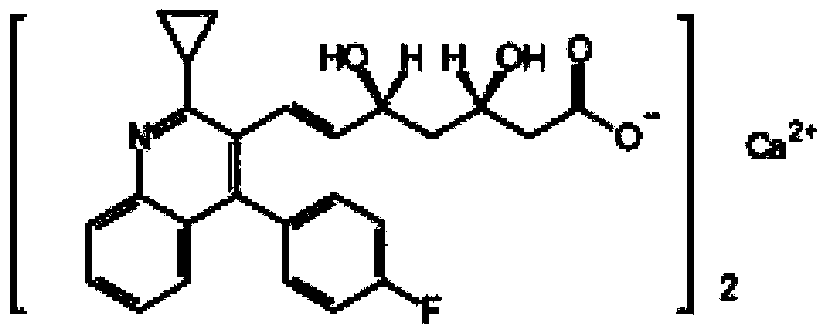
Stable pitavastatin calcium pharmaceutical composition and preparation method thereof
A technology of pitavastatin calcium and its composition, which is applied in the field of stable pharmaceutical composition and its preparation, can solve the problems of product stability, uneven tablet color, and color change of magnesium oxide, and achieve good stability, To avoid drug degradation, the effect of simple preparation process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
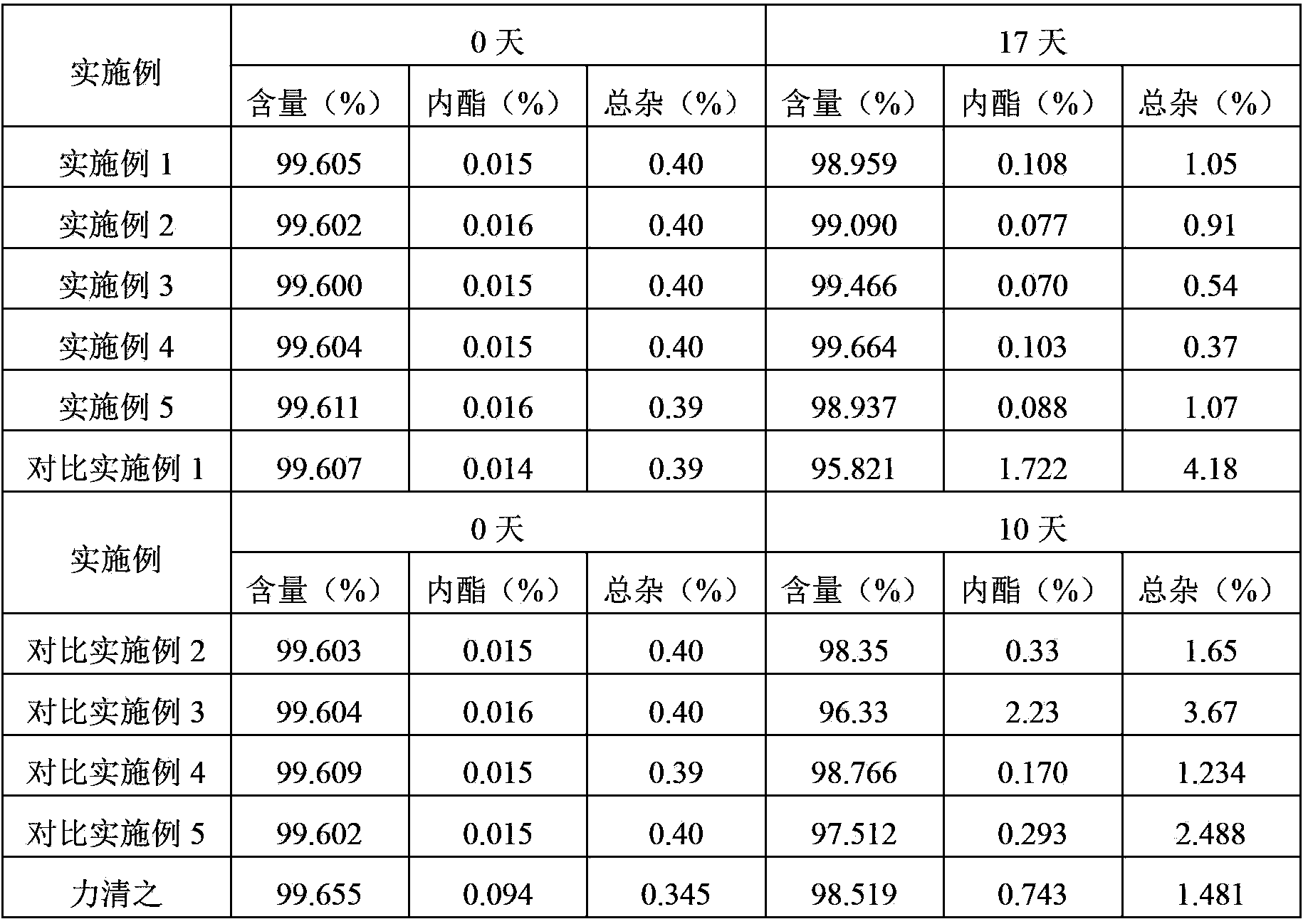
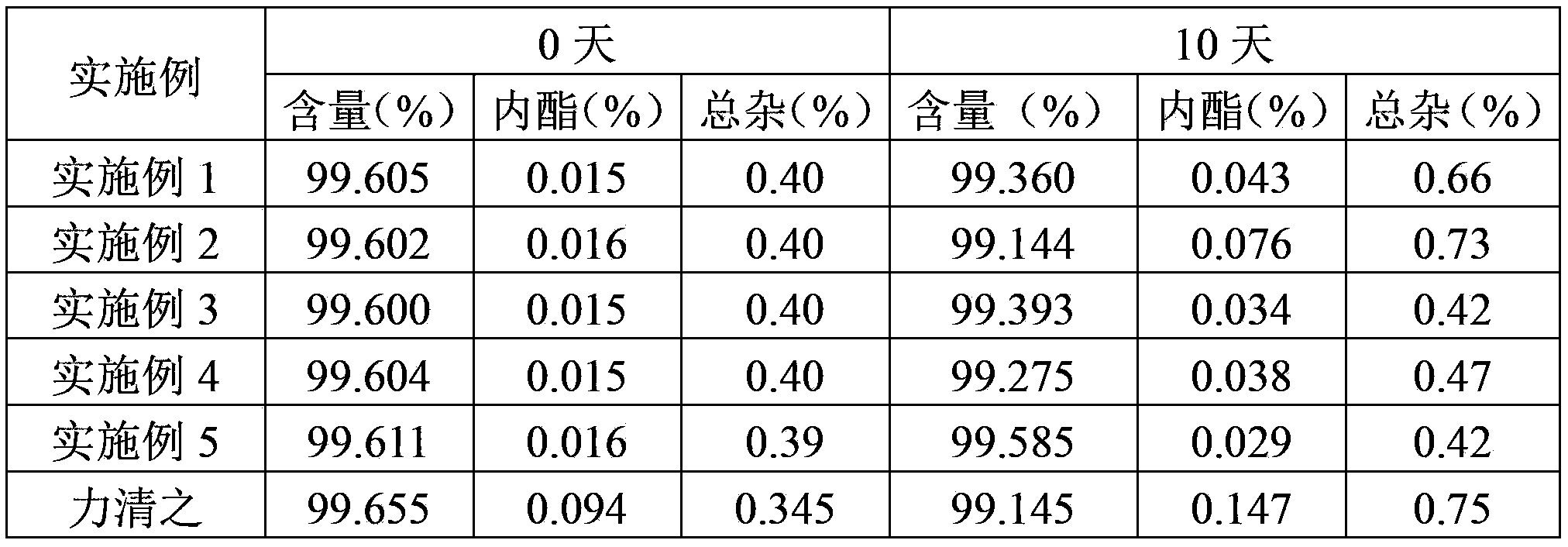
Examples
Embodiment 1
[0032] Element
[0033] Pass the crude drug and each auxiliary material in the above composition through an 80-mesh sieve for later use. Add the prescribed amount of pitavastatin calcium and red iron oxide in equal amounts and mix well, add the prescribed amount of microcrystalline cellulose, and then fully mix for 30 minutes, then add the prescribed amount of magnesium stearate, and continue mixing for 5 minutes, Obtain a homogeneous mixed powder. The obtained homogeneous mixed powder was pressed into 1000 tablets.
Embodiment 2
[0035] Element
[0036] Pass the crude drug and each auxiliary material in the above composition through an 80-mesh sieve for later use. Add the prescribed amount of pitavastatin calcium and aluminum oxide in equal increments and mix well, then add the prescribed amount of microcrystalline cellulose and hydroxypropyl cellulose in turn, and mix thoroughly for another 30 minutes, then add the prescribed amount of magnesium stearate , and continue to mix for 5 minutes to obtain a uniform mixed powder. The obtained homogeneous mixed powder was pressed into 1000 tablets.
Embodiment 3
[0038] Element
[0039] Pass the crude drug and each auxiliary material in the above composition through an 80-mesh sieve for later use. Add the prescribed amount of pitavastatin calcium and yellow iron oxide in equal increments and mix well, then add the prescribed amount of microcrystalline cellulose and hydroxypropyl cellulose in turn, and mix thoroughly for 30 minutes, then add the prescribed amount of stearic acid Magnesium, continue mixing for 5 minutes to obtain a homogeneous mixed powder. The obtained homogeneous mixed powder was pressed into 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com