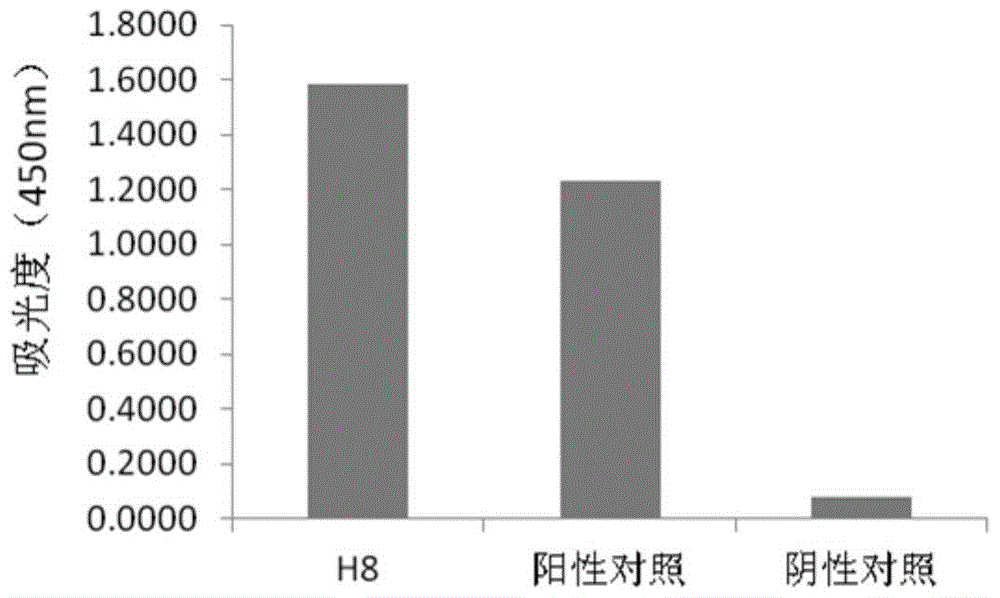
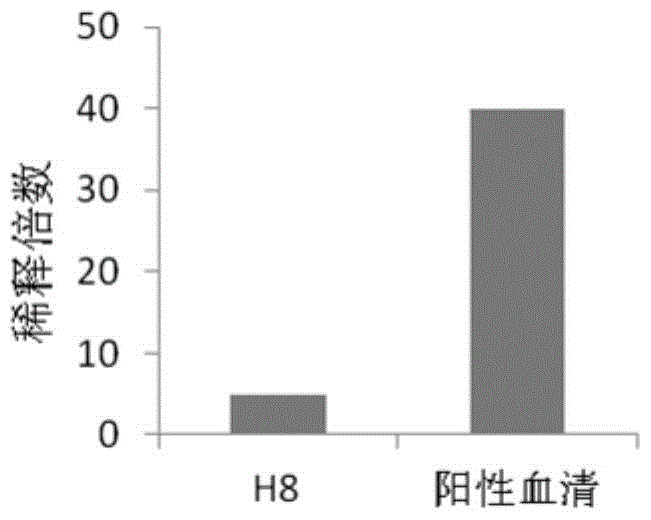
Human anti-h7n9 avian influenza virus neutralizing antibody h8, its preparation method and application
A bird flu virus and antibody technology, applied in antiviral agents, antiviral immunoglobulin, botanical equipment and methods, etc., can solve problems such as restrictions, blood-borne disease infection, and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1 Materials and methods
[0033]1.1 Source of virus, cell and vector: H7N9 virus A / Anhui / 1 / 2013 strain has been published in the literature (Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L ,Guo J,Dong J,Li Q,Dong L,Zhu Y,Bai T,Wang S,Hao P,Yang W,Zhang Y,Han J,YuH,Li D,Gao GF,Wu G,Wang Y,Yuan Z , Shu Y.2013.Human infection with a novelavian-origin influenza A(H7N9)virus.N Engl J.Med.368:1888–1897), provided by the Institute of Pathogenic Biology, Chinese Academy of Medical Sciences. The cells used for the in vitro neutralization experiment of H7N9 avian influenza virus were MDCK cells, which were purchased from ATCC. The bacterial strain used to construct the phage antibody library was XL1-Blue (Stratagene, USA), and the vector pComb3H (provided by Scripps Research Institute, USA).
[0034] 1.2 Antigen preparation
[0035] ...
Embodiment 2
[0104] Example 2 Application of human-derived anti-H7N9 avian influenza virus neutralizing antibody H8
[0105] At present, there are no specific vaccines and drugs for the prevention and treatment of acute respiratory infectious diseases caused by the H7N9 virus. The immunoglobulins used clinically are often derived from the convalescent serum of patients infected with the virus, which limits their mass production. At the same time, because it comes from serum, it is prone to infection of blood-borne diseases. The invention provides a new approach for the treatment of acute respiratory infectious diseases caused by the H7N9 virus by using the human source anti-H7N9 avian influenza virus genetically engineered antibody H8 to replace the blood-derived immunoglobulin.
Embodiment 3
[0106] Example 3 The method for preparing full antibody immunoglobulin IgG by using neutralizing antibody H8
[0107] 1. Expression and purification of whole antibody IgG
[0108] ① Construction of whole antibody recombinant expression plasmid: first use primers (upstream primer 5'-CCCAAGCTTGTTGCTCTGGATCTCTGGTGCCTACGGGGACATCCAGATGACCCAGTCTCCATCCTCC-3', downstream primer 5'-CTAGTCTAGAATTAACACTCTCCCCTG-3') to amplify the light chain segment of the Fab antibody, digest with XbaI / HindIII, Cloned into PIgG vector (provided by Scripps Research Institute, USA) (Christoph Rader, Mikhail Popkov, JohnA.Neves, and Carlos F.Barbas III. Integrinαvβ3-targeted therapy for Kaposi.ssarcoma with an in vitro-evolved antibody. The FASEB Journal.( October 18, 2002) 10.1096 / fj.02-0281fje.), the vector PIgG-L was obtained. Then use primers (upstream primer 5'-GAGGAGCTCACTCCAGGTGCAGCTGCAGGAGTCGGGCCCAGGAC-3', downstream primer 5'-GAGGGGCCCTTGGTGGAGGCTGAGGAGACGGT-3') to amplify the heavy chain segment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com