Preparation method and application of pseudolarix lactone H
A technology of pinolactone and golden pine, which is applied in the field of medicine, can solve the problems of insulin dependence, limited application, and large toxic and side effects, and achieve the effects of improving crystal uniformity, accelerating production efficiency, and reducing impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of chrysanthemum lactone H
[0036] After crushing 12 kg of pine seeds, reflux extraction with 50 liters of dichloromethane at 60°C for 12 hours, after standing still, filter the dichloromethane solution and pine seed raw material powder (filtrate), and concentrate the obtained dichloromethane solution Dry to obtain the concentrated extract A for later use, then reflux the filtered pine seed powder with 50 liters of ethyl acetate at 80°C for 12 hours, filter to obtain the ethyl acetate solution, concentrate and dry the ethyl acetate solution to obtain the concentrated extract B standby. The aforementioned concentrated extract A and concentrated extract B were mixed, and dispersed in 45 liters of methanol-water (volume ratio 5:1) mixed solution, extracted and degreased twice with 45 liters of petroleum ether, and the raffinate was concentrated and dried to obtain 45g of pine seed triterpene extract, wherein the content of triterpenoids meas...
Embodiment 2
[0037] Embodiment 2: the preparation of chrysanthemum lactone H
[0038] After crushing 100 kg of pine seeds, reflux extraction with 300 liters of chloroform at 60°C for 12 hours, after standing still, filter the chloroform solution and pine seed raw material powder (filtrate), and concentrate the chloroform solution Dried to obtain concentrated extract A for later use, then reflux extracted the filtered pine seed powder with 300 liters of acetone at 60°C for 12 hours, filtered to obtain an acetone solution, concentrated and dried the acetone solution to obtain concentrated extract B for later use. The aforementioned concentrated extract A and concentrated extract B were mixed, and dispersed in 300 liters of methanol-water (volume ratio 10:1) mixed solution, extracted and degreased twice with 300 liters of petroleum ether, and the raffinate was concentrated and dried to obtain 351g of the triterpene extract of pine seed, the content of triterpenoids is 58.1% as measured by ult...
Embodiment 3
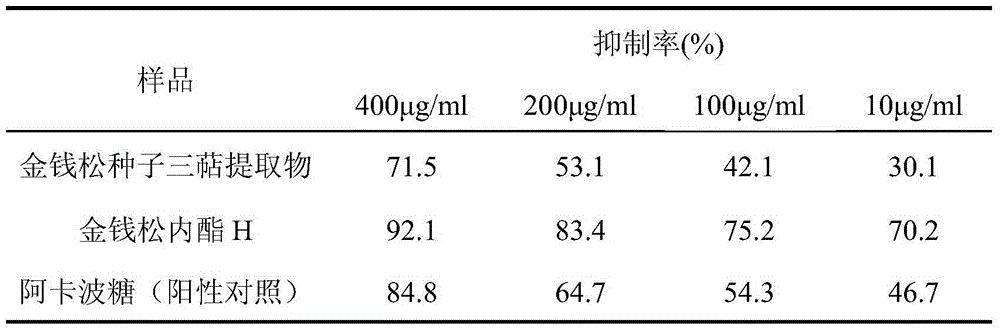
[0039] Example 3: Test of α-glucosidase inhibitory activity of triterpene extract and its component chrysanthemum lactone H in pine seeds
[0040] α-glucosidase was used to catalyze the hydrolysis of p-nitrophenyl-α-D-glucopyranoside, and the activity of α-glucosidase was determined by measuring the amount of p-nitrophenol released. The enzyme inhibitory activity of the extract was calculated based on the content change of p-nitrophenol in the reaction system within a certain period of time. The specific results are shown in Table 1. The results showed that the triterpene extracts of pine seeds had significant inhibitory activity on α-glucosidase in vitro, which was similar to that of acarbose, while the hypoglycemic effect of chrysanthemum lactone H was better than that of acarbose.
[0041] Table 1 Inhibitory activity of pine seed triterpene extract on α-glucosidase
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com