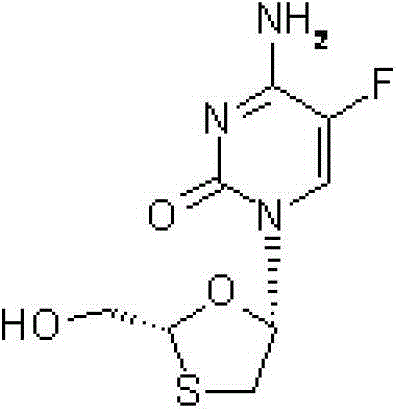
Emtricitabine-containing tablet and preparation method thereof
A technology for emtricitabine and tablets, which is applied in the field of drug preparation, can solve the problems of affecting tablet stability, release of active ingredients, instability of acids, bases and oxidants, incomplete suitability for AIDS patients, etc., and achieves easy industrial production, Accurate dosage and high dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
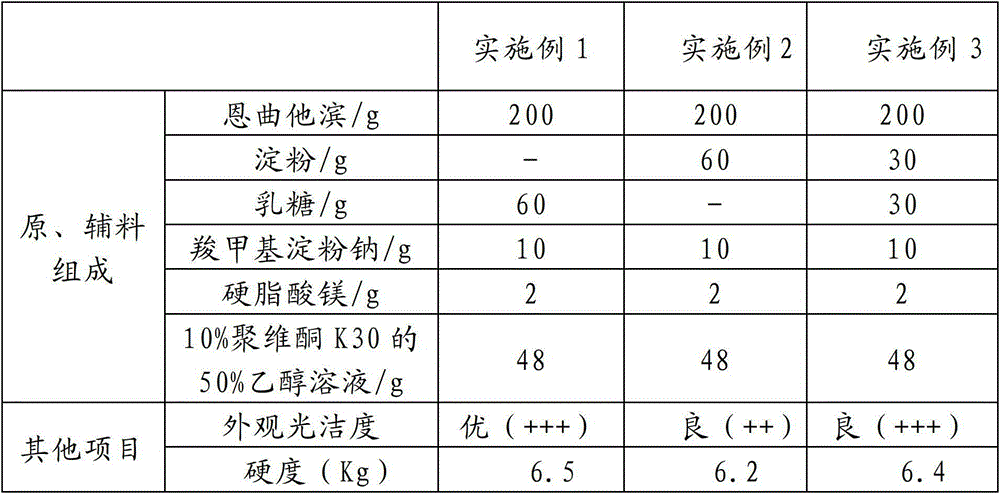
[0045] The proportioning ratio of raw materials and auxiliary materials in Examples 1 to 3 is shown in Table 1, and the preparation methods are basically the same, and reference can be made to the preparation method in Example 1 below.
[0046] Table 1
[0047]
[0048] The preparation method of the tablet containing emtricitabine in embodiment 1 comprises the following steps:
[0049] (a) The preparation method of the adhesive (50% ethanol solution of 10% povidone K30) is as follows: dissolve 4.8 g of povidone K30 in 43.2 g of 50% ethanol aqueous solution, stir until dissolved, and set aside.
[0050] (b) Preparation of mixture granules: as shown in Table 1, add the required amount of emtricitabine, lactose and carboxymethyl starch sodium into the wet mixing granulator, stir and mix for 400 seconds, and wait until the mixture is uniform , add 48g of 10% povidone K30 in 50% ethanol solution to the granulator, wet granulate for 90 seconds, and dry at 60°C until the water co...
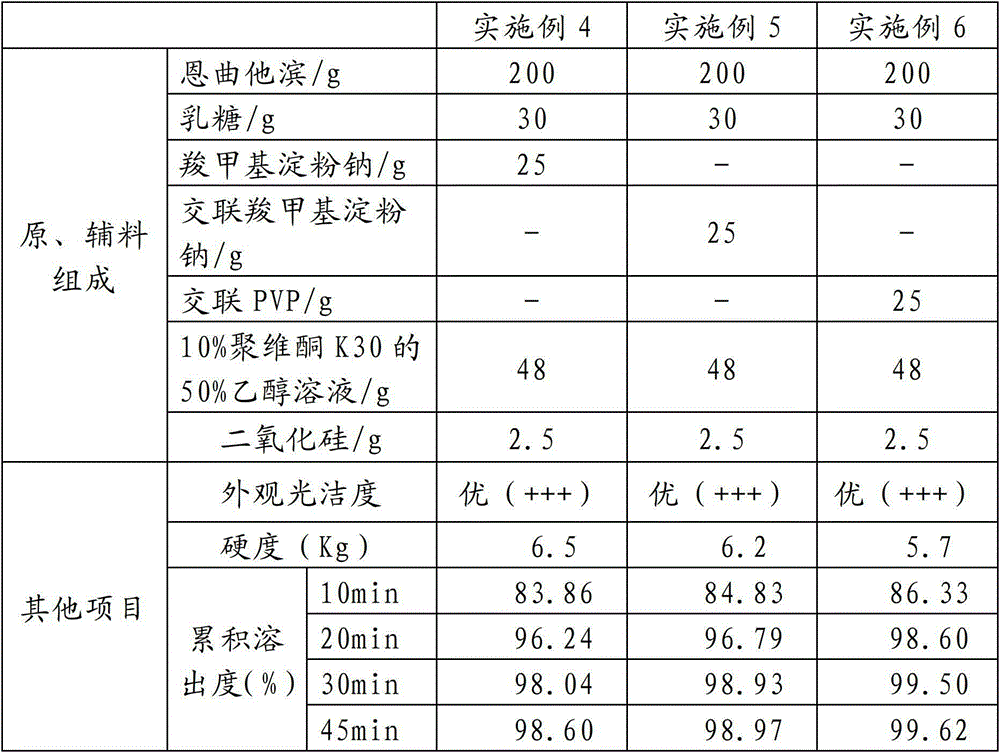
Embodiment 4~6
[0053] Examples 4 to 6 are as shown in Table 2. For the preparation method, refer to the preparation method of Example 1, wherein in step (2), after 60 seconds of wet granulation, dry at 50°C.
[0054] Table 2
[0055]
Embodiment 7~10
[0057] The proportioning ratio of raw materials and auxiliary materials in Examples 7-10 is shown in Table 3. For the preparation method, refer to the preparation method of Example 1, wherein in step (2), after wet granulation for 120 seconds, dry at 70°C.
[0058] table 3
[0059]
[0060] Long-term Stability Test
[0061] The sample of the emtricitabine-containing tablet prepared in Example 1 was subjected to a long-term stability test at room temperature, and the results are shown in Table 4.
[0062] Table 4
[0063] time
character
tablet weight difference
Dissolution (%)
Content (%)
relative substance(%)
0 months
Compliance
Compliance
99.98
95.98
0.42
March
Compliance
Compliance
101.97
95.70
0.51
June
Compliance
Compliance
100.79
96.26
0.34
September
Compliance
Compliance
99.12
95.56
0.42
December
Compliance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com