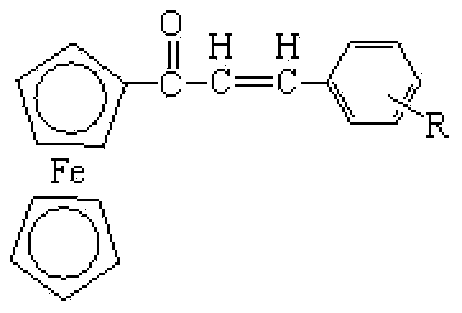
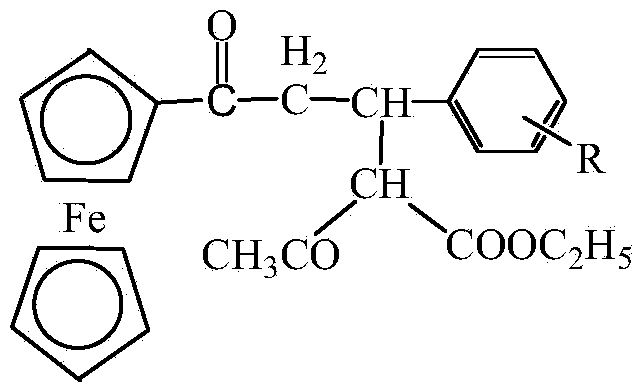
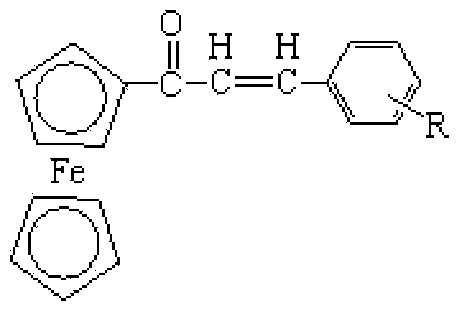
Michael addition product of ferrocene-based chalcone and ethyl acetoacetate and preparation method thereof
A technology of ethyl acetoacetate and ethyl acetoacetate is applied in the field of preparing the Michael addition product of ferrocenyl chalcone and ethyl acetoacetate, which can solve the environmental pollution of organic solvents, long reflux reaction time and product purification. Trouble and other problems, to achieve the effect of less environmental pollution, convenience and easy operation, and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 0.05 mol ferrocenylphenylchalcone ( be phenyl), 0.05mol ethyl acetoacetate and 0.0005mol sodium hydroxide, grind at room temperature until the raw material reacts completely, at this moment, TLC monitors and finds that the raw material point of ferrocenyl phenyl chalcone disappears (the developer of TLC is A mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3), then the product obtained by the reaction is washed with water, suction filtered, and the filter cake is recrystallized with water to obtain ferrocenyl phenyl chalcone and acetoacetic acid The Michael addition product of ethyl ester ( is phenyl), the yield is 91.3%, and the melting point is 178-185°C.
[0030] IR (KBr tablet): 3104cm -1 (Unsaturated C-H); 2922cm -1 ,2853cm -1 (saturated C-H); 1712cm -1 (-C=O-); 1596cm -1 、1573cm -1 、1494cm -1 (breathing vibration of benzene ring); 1375cm -1 、1456cm -1 (methyl and methylene); 718cm -1 、753cm -1 (monosubstituted benzene r...
Embodiment 2
[0033] Add 0.5 mol ferrocenyl-p-methylphenylchalcone ( be p-methylphenyl), 0.5mol ethyl acetoacetate and 0.006mol sodium hydroxide, grind at room temperature until the raw material reacts completely, at this time TLC monitors and finds that the raw material point of ferrocenyl p-methylphenylchalcone disappears (The developer of TLC is the mixed solvent of ethyl acetate and sherwood oil that the volume ratio is 1:3), then the product obtained by the reaction is washed with water, suction filtered, and the filter cake is recrystallized with water to obtain the ferrocenyl p-formaldehyde The Michael addition product of phenylchalcone and ethyl acetoacetate ( is p-methylphenyl), the yield is 92.4%, and the melting point is 178-183°C.
[0034] IR (KBr tablet): 3107cm -1 (Unsaturated C-H); 2989cm -1 ,2856cm -1 (saturated C-H); 1713cm -1 (-C=O-); 1587cm -1 、1513cm -1 、1451cm -1 (breathing vibration of benzene ring); 1375cm -1 、1443cm -1 (methyl and methylene); 819cm -1 (p...
Embodiment 3
[0037] Add 0.5 mol ferrocenyl p-methoxyphenylchalcone ( For p-methoxyphenyl), 0.5mol ethyl acetoacetate and 0.007mol sodium hydroxide, ground at room temperature until the raw material reacted completely, at this time TLC monitoring found the raw material of ferrocenyl p-methoxyphenyl chalcone point disappears (the developer of TLC is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3), and then the product obtained by the reaction is washed with water, suction filtered, and the filter cake is recrystallized with water to obtain ferrocenyl The Michael addition product of p-methoxyphenylchalcone and ethyl acetoacetate ( is p-methoxyphenyl), the yield is 91.7%, and the melting point is 199-204°C.
[0038] IR (KBr tablet): 3149cm -1 (Unsaturated C-H); 2971cm -1 ,2831cm -1 (saturated C-H); 1712cm -1 (-C=O-); 1583cm -1 、1510cm -1 、1456cm -1 (breathing vibration of benzene ring); 1375cm -1 、1432cm -1 (methyl and methylene); 827cm -1 (para-po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com