Mesoporous titanium dioxide material and preparation method thereof
A technology of mesoporous titanium dioxide and pore size, which is applied in the field of mesoporous materials, can solve the problems of insufficient environmental protection of the preparation system, complex preparation system, and lengthy process, and achieve the effects of stable room temperature, simple control process, and simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] There are many methods for preparing titanic acid. Among them, the method of preparing titanic acid by reacting the initial titanium source with water or using titanyl sulfate as the initial titanium source is relatively simple, and the reaction conditions are easy to control. Specifically, the initial titanium source includes four Any one or more of titanium chloride, titanium trichloride, and titanates;
[0044] The initial titanium source reacts with water to produce titanic acid through hydrolysis and condensation;
[0045] The molar ratio of the initial titanium source to water is 5-10:1.
[0046] More preferably, the initial titanium source is titanium tetrachloride. Titanates include tetrabutyl orthotitanate, tetraisopropyl titanate, ethyl titanate, methyl titanate, and the like.
[0047] Preferably, the initial titanium source is titanyl sulfate, and the titanyl sulfate is neutralized with ammonia water to a pH value of 6-8 to obtain a precipitate, which is wa...
Embodiment 1
[0053] Titanyl sulfate is neutralized with ammonia water to a pH value of 7 to obtain a precipitate, which is washed and centrifuged to obtain titanic acid;
[0054] Titanic acid and hydrogen peroxide react in water to prepare peroxide titanic acid sol, and the molar ratio between titanic acid, hydrogen peroxide and water is 1:15:120;
[0055] P123(EO 20 PO 70 EO 20 ) Surfactant is added to peroxotitanic acid sol solution, and mixed to obtain a self-assembly system, the molar ratio of surfactant to titanic acid is 0.03:1;
[0056] The self-assembly system volatilizes its own liquid, and the volatilization temperature is 100°C to obtain a composite material of peroxotitanic acid and surfactant;
[0057] The composite material was heated at a heating rate of 2° C. / min, and fired at 450° C. for 2 hours to obtain a mesoporous titanium dioxide material.
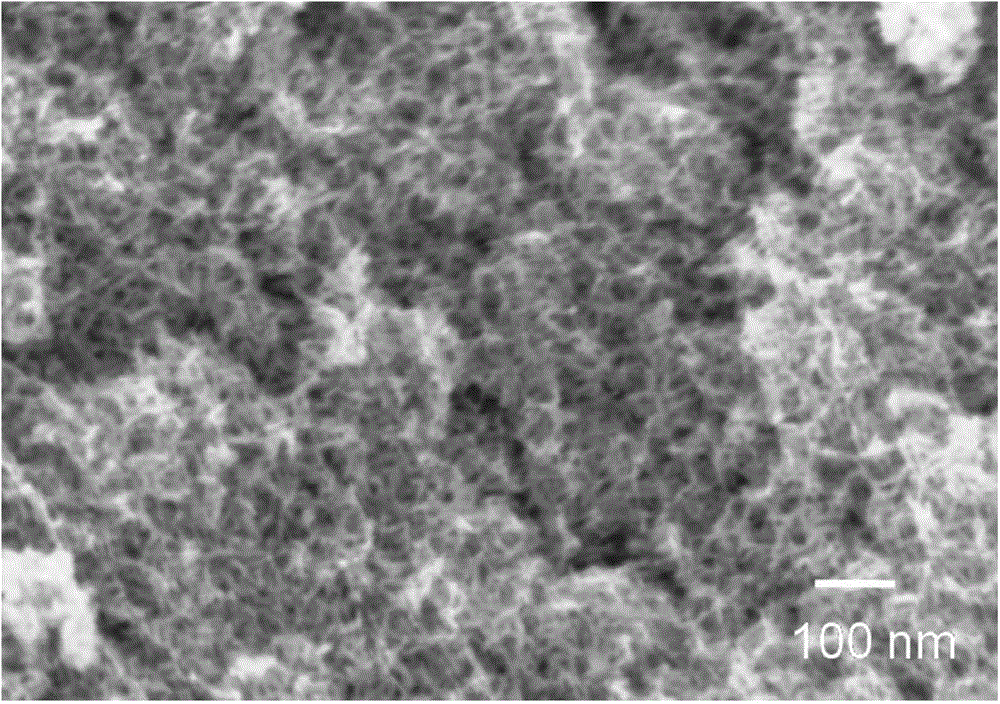
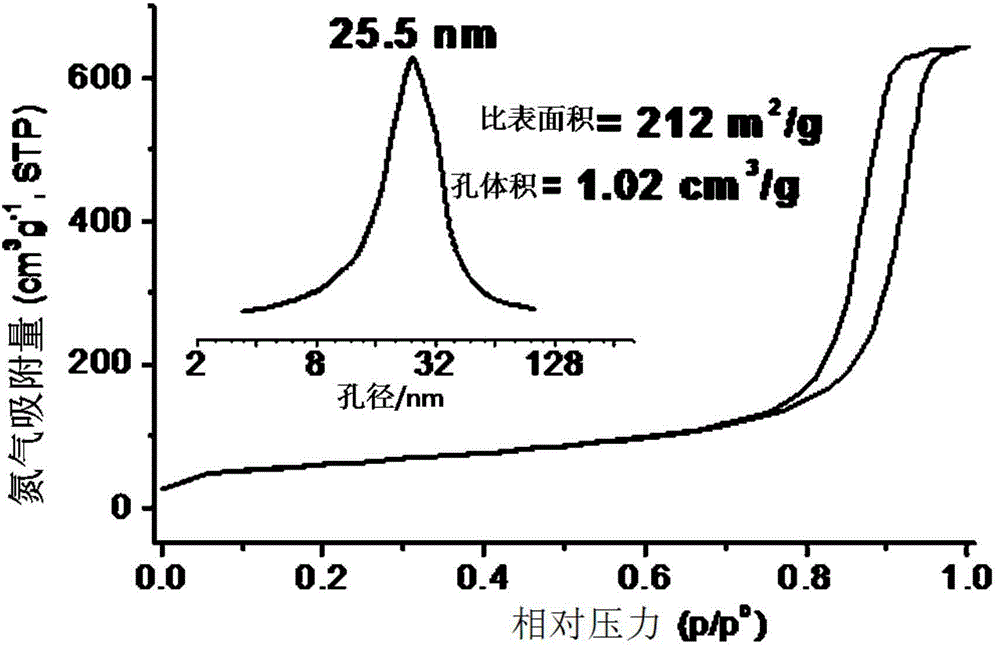
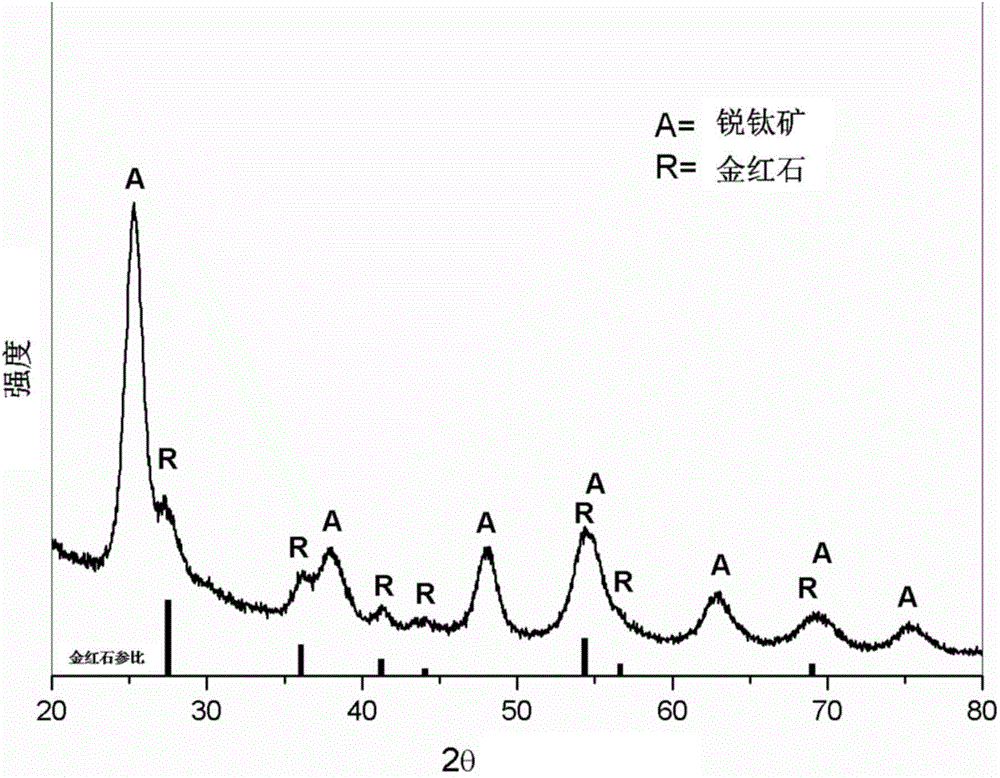
[0058] The mesoporous structure of the obtained mesoporous titanium dioxide material can be seen by scanning electron micros...
Embodiment 2
[0060] The molar ratio of titanium trichloride and water is 7:1 to react to produce titanic acid;
[0061] Titanic acid and hydrogen peroxide react in water to prepare peroxide titanic acid sol, the molar ratio between titanic acid, hydrogen peroxide and water is 1:15:100;
[0062] Polyoxyethylene-polyoxybutylene (EO 10 BO 18 ) Surfactant is added to peroxotitanic acid sol solution, and mixed to obtain a self-assembly system, the molar ratio of surfactant to titanic acid is 0.01:1;
[0063] The self-assembly system volatilizes its own liquid, and the volatilization temperature is 50°C to obtain a composite material of peroxotitanic acid and surfactant;
[0064] The composite material was heated at a heating rate of 3°C / min, and fired at 450°C for 4 hours to obtain a mesoporous titanium dioxide material.
[0065] The mesoporous structure of the obtained mesoporous titanium dioxide material can be seen by scanning electron microscopy, and its three-dimensional interconnected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com