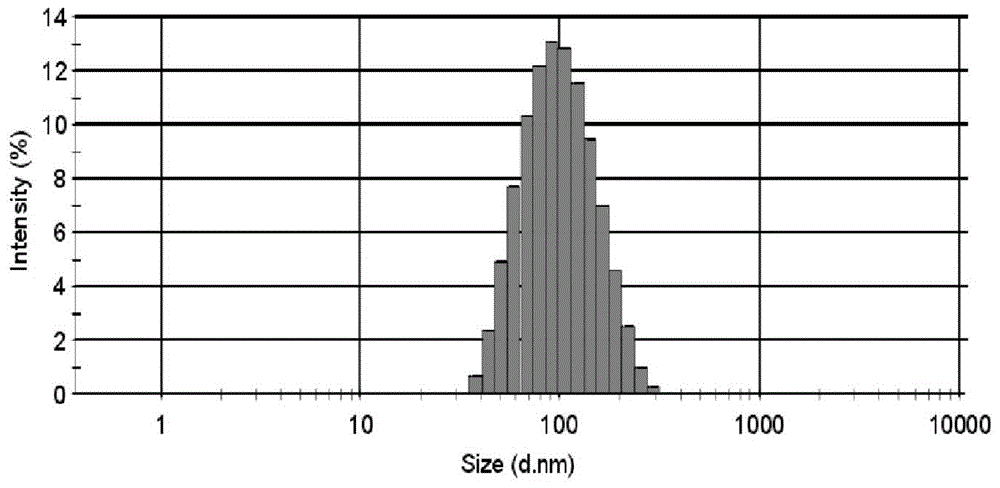
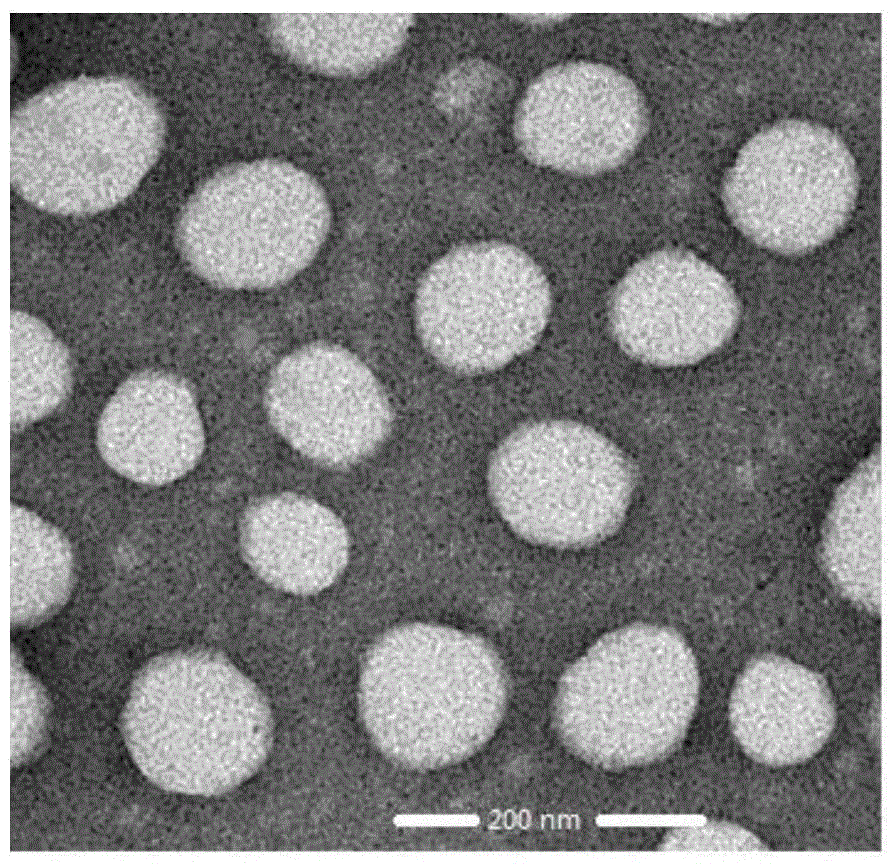
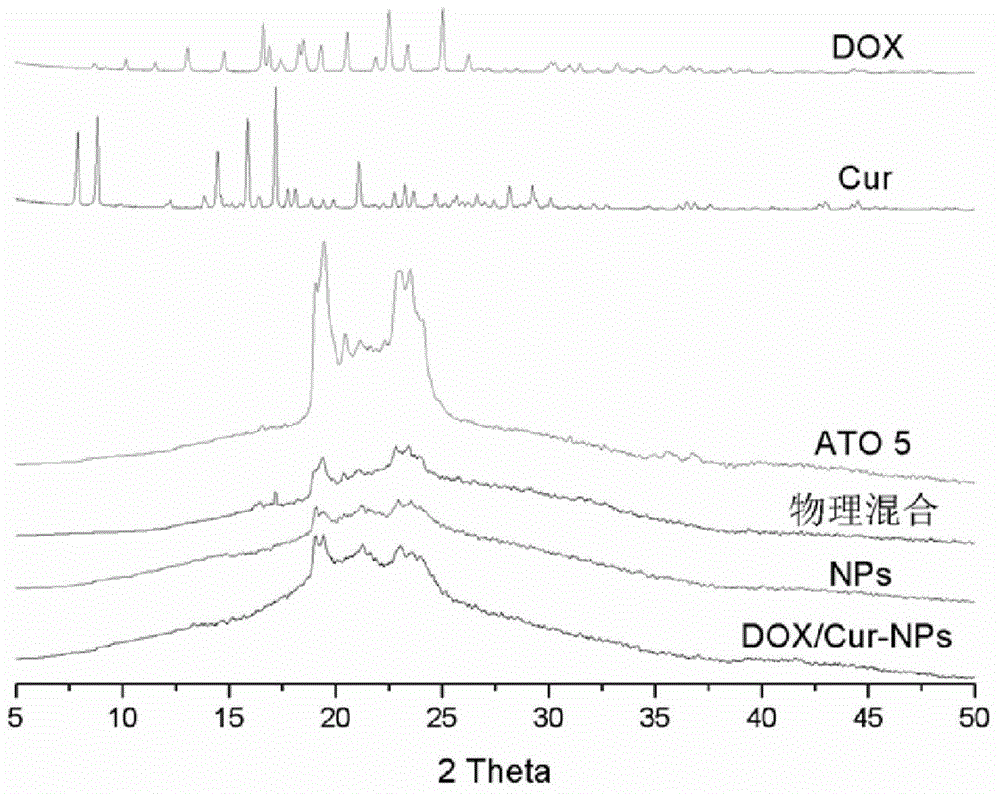
Adriamycin nano drug delivery system as well as preparation method and application thereof
A nano-loaded drug and doxorubicin technology, which is applied in the direction of pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve the problems of inability to deliver two active ingredients at the same time, inconsistent pharmacokinetic behavior, and low bioavailability of drugs , achieve good market development prospects, enhance anti-cancer effects, and have no obvious toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the doxorubicin nano-loading system provided by the invention comprises the following steps:
[0053] (1) Uniformly disperse the doxorubicin-containing active ingredient, solid lipid, liquid lipid and fat-soluble emulsifier in the organic solvent in the formula ratio, evaporate the organic solvent to dryness at 65°C to 80°C to obtain an oil phase, and maintain the temperature.
[0054] The fat-soluble emulsifier, such as phospholipids and the like.
[0055] The doxorubicin is fat-soluble doxorubicin, which can be obtained as follows: dissolve the water-soluble doxorubicin hydrochloride in an organic solvent, and add triethylamine 2 to 5 times the molar mass of doxorubicin hydrochloride for neutralization , stirred overnight in the dark, and the solvent was spun to dryness to obtain fat-soluble doxorubicin.
[0056] The organic solvent is dichloromethane, chloroform or ethanol, preferably absolute ethanol.
[0057] (2) Uniformly disperse other...
Embodiment 1
[0068] Doxorubicin nano-loading system, according to the mass ratio, includes 1.5% active ingredient containing doxorubicin, 20% solid lipid, 20% liquid lipid, 5% emulsifier and 5% isotonic regulator, and the balance is injection use water.
[0069] The active ingredient containing doxorubicin is a 1:10 mixture of doxorubicin and a sensitizer, and the sensitizer is curcumin.
[0070] The emulsifier contains polyethylene glycol 5000 vitamin E succinate with 10% total mass of emulsifier, and the emulsifier also contains polyoxyethylene-polyoxypropylene block copolymer with 90% total mass of emulsifier
[0071] The solid lipid comprises glyceryl monostearate with 100% of the total mass of the solid lipid.
[0072] The liquid lipid comprises 50% glyceryl linoleate and 50% isopropyl myristate of the total mass of the liquid lipid.
[0073] The isotonic regulator includes 100% glucose in the total mass of the isotonic regulator.
[0074] The solid lipid and the liquid lipid form ...
Embodiment 2
[0076] Doxorubicin nano-loading system, according to the mass ratio, includes 0.02% active ingredient containing doxorubicin, 1% solid lipid, 0.2% liquid lipid, 10% emulsifier and 0.5% isotonic regulator, and the balance is injection use water.
[0077] The active ingredient containing doxorubicin is a 1:1 mixture of doxorubicin and a sensitizer, and the sensitizer is curcumin.
[0078] The emulsifier contains polyethylene glycol 2000 vitamin E succinate with 5% of the total mass of the emulsifier, and the emulsifier also contains polysorbate with 95% of the total mass of the emulsifier.
[0079] The solid lipid comprises 100% stearic acid of the total mass of the solid lipid.
[0080] The liquid lipid comprises 100% propylene glycol monocaprylate of the total mass of the liquid lipid.
[0081] The isotonic regulator includes 100% glycerol in the total mass of the isotonic regulator.
[0082] The solid lipid and the liquid lipid form nanoparticles under the action of the em...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com