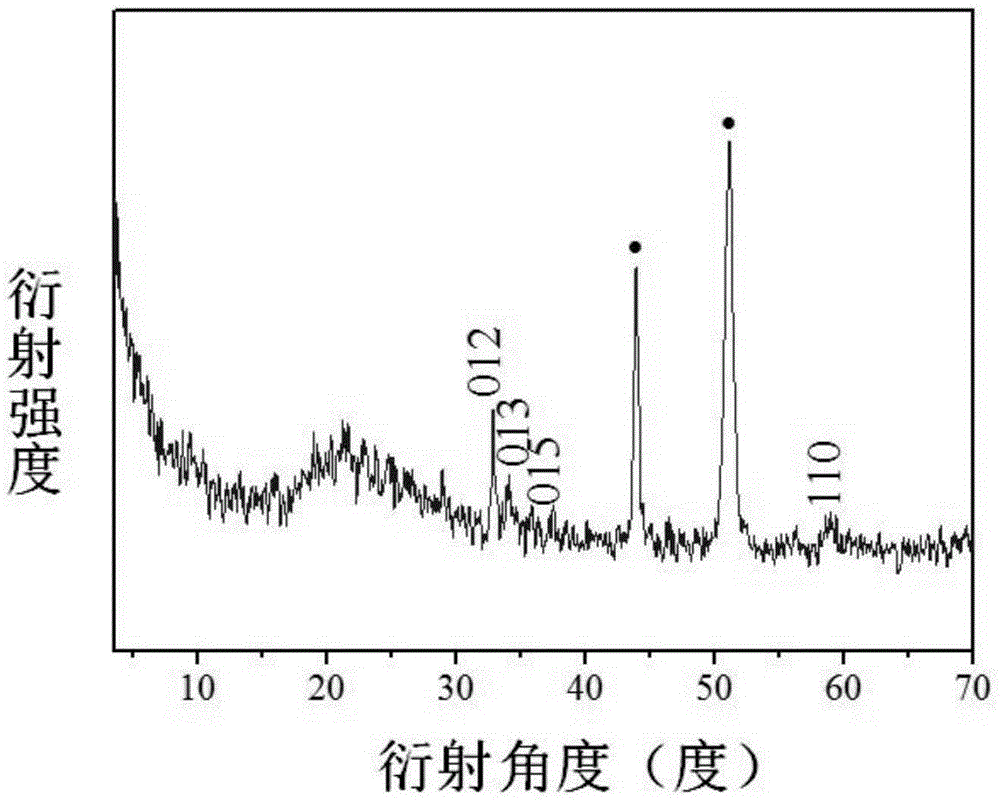
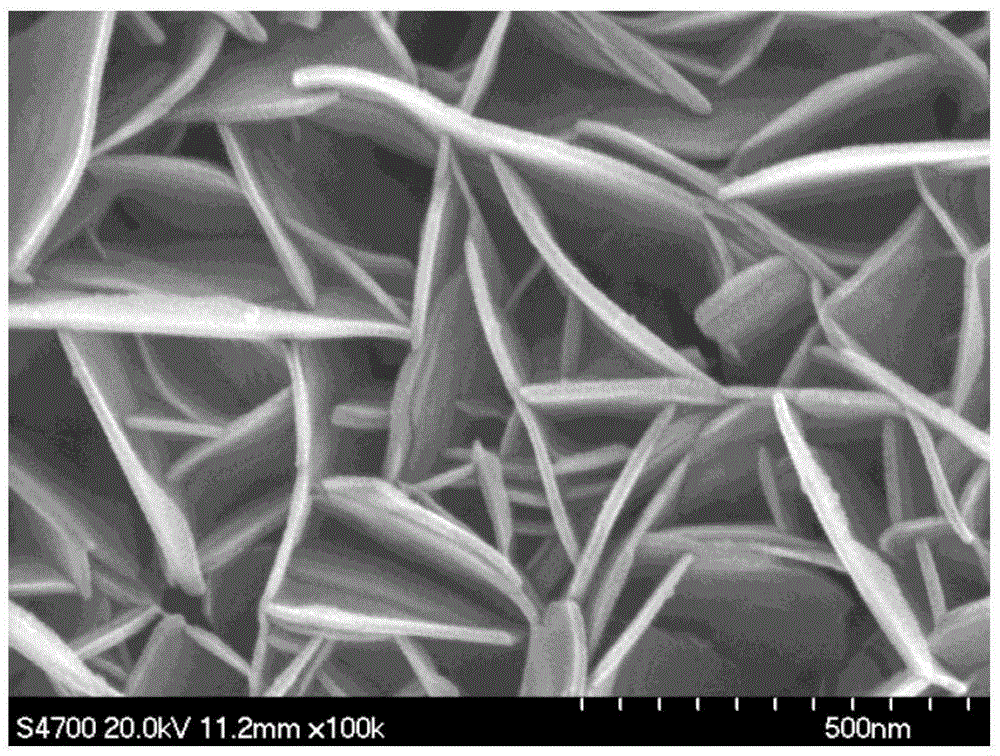
Metal substrate/cobalt-containing hydrotalcite nano-membrane electrode and preparation method thereof
A technology based on metal substrates and hydrotalcites, applied in electrodes, electrolytic components, electrolytic processes, etc., can solve the problems of weak binding force between catalyst powder and electrode matrix, dispersion of powder samples, and decreased activity, and achieve high catalytic water splitting oxidation activity and long-term stability, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.4735g of zinc nitrate hexahydrate and 0.7275g of cobalt nitrate hexahydrate, dissolve them in 100mL of deionized water to prepare a mixed salt solution, and add 0.19mL of H 2 o 2 , the resulting solution was blown with nitrogen for 1 hour, and transferred to a single-chamber electrolytic cell, where the working electrode had an area of 1 cm 2 , Nickel foil with a thickness of 0.03mm, the counter electrode is a platinum wire electrode, the reference electrode is an Ag / AgCl electrode, connected to the electrochemical workstation, and a potential of -1V (to the Ag / AgCl electrode) is applied to the nickel foil electrode for electrodeposition. After deposition for 100 seconds, the nickel foil was taken out, thoroughly washed with deionized water, and dried in an oven at 70°C for 0.5 hours.
[0030] Evaluation of catalytic performance: Weigh 2.8055g of potassium hydroxide, dissolve it in 50mL of deionized water and transfer it to the electrolytic cell, take the nic...
Embodiment 2
[0032] Weigh 1.1502g of zinc sulfate heptahydrate and 0.2811g of cobalt sulfate heptahydrate, dissolve them in 100mL of deionized water to prepare a mixed salt solution, and add 0.19mL of H 2 o 2 , the resulting solution was passed through nitrogen for 1 hour, and transferred to a single-chamber electrolytic cell, where the working electrode had an area of 5 cm 2 , a nickel foil with a thickness of 0.03mm, the counter electrode is a platinum wire electrode, and the reference electrode is an Ag / AgCl electrode. Electrodeposition was performed at the potential of V (for the Ag / AgCl electrode), and the deposition time was 120 seconds. After the deposition, the nickel foil was taken out, fully washed with deionized water, and dried in an oven at 70°C for 0.5 hours.
[0033] The catalytic performance evaluation method is the same as above, and the evaluation results: the overpotential of water oxidation to generate oxygen is 0.34V, and the reaction current density at a potential ...
Embodiment 3
[0035] Weigh 5.3917g of zinc sulfate heptahydrate and 1.7569g of cobalt sulfate heptahydrate, dissolve them in 50mL of deionized water to prepare a mixed salt solution, and add 0.94mL of H 2 o 2 , the resulting solution was passed through nitrogen for 1 hour, transferred to a single-chamber electrolytic cell, and a three-electrode electrolytic cell was built, in which the working electrode had an area of 2 cm 2 , a nickel foil with a thickness of 0.12mm, the counter electrode is a platinum wire electrode, and the reference electrode is an Ag / AgCl electrode, connected to an electrochemical workstation (the electrodes and workstation used are purchased from Shanghai Chenhua Instrument Co., Ltd.), and -1V is applied to the nickel foil. (Ag / AgCl electrode) potential for electrodeposition, the deposition time is 150 seconds, the nickel foil is taken out after the deposition, fully washed with deionized water, and dried in an oven at 70 ° C for 0.5 hours.
[0036] The catalytic p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com