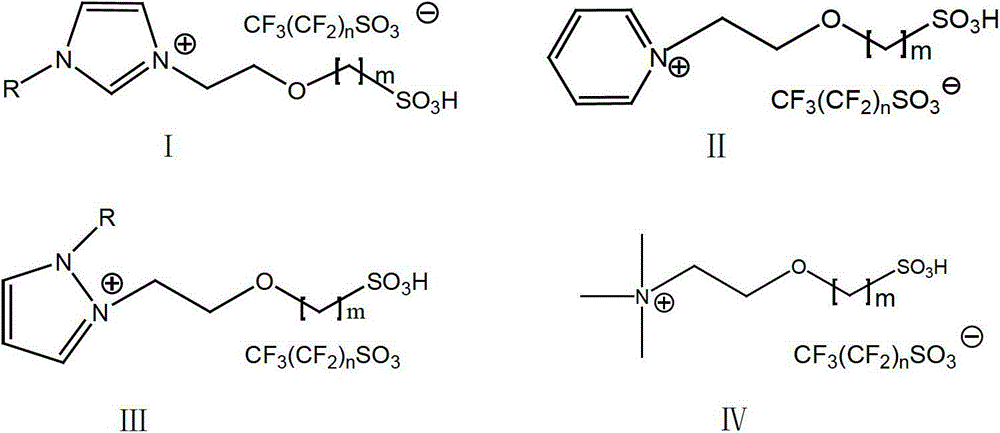
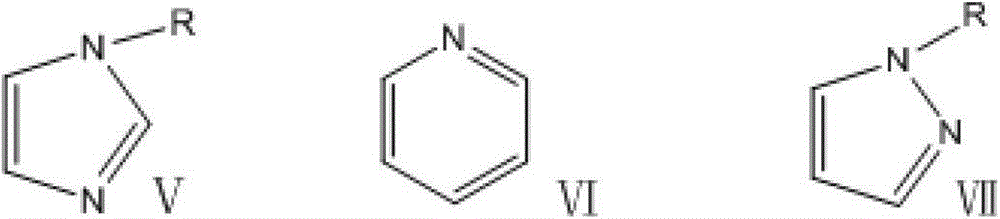
Sulfonic acid functionalized ionic liquid based on perfluoro alkyl sulfonic acid radical negative ion and its preparation method
A perfluoroalkane sulfonate and perfluoroalkane sulfonate technology, which is applied in the fields of sulfonic acid preparation, sulfonate preparation, organic chemistry, etc., can solve the "designable" space limitation and low activity of catalytic esterification reaction , Inconvenient operation process and other problems, to achieve good industrial application prospects, lower viscosity, reduce the effect of difficult operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
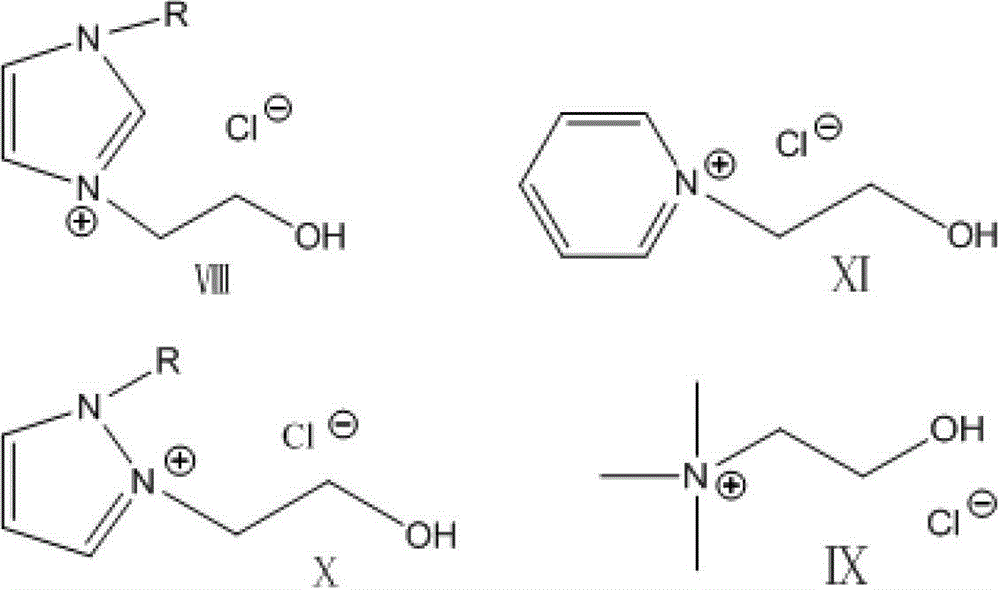
[0030] The preparation of embodiment 1.3-(2-hydroxyethyl)-1-methylimidazolium chloride:
[0031]
[0032] Put 1.0mol methylimidazole into a 500mL three-necked flask, stir and drop 1.0mol chloroethanol at 80°C, and react at a constant temperature for 12 hours after the addition is completed. After the reaction mixture is cooled to room temperature, a white or light yellow jelly-like substance can be obtained. Wash and filter with anhydrous ether three times, and dry in vacuum for 8 hours to obtain a white or light yellow solid, dry in vacuum at 80°C for 24 hours.
Embodiment 2
[0033] The preparation of embodiment 2.N-hydroxyethylpyridine chloride:
[0034]
[0035] Put 1.0mol of pyridine into a 500mL three-necked flask, stir and add 1.0mol of chloroethanol dropwise at 80°C. After the dropwise addition, keep it warm for 12 hours. After the reaction mixture is cooled to room temperature, a white or light yellow jelly-like substance can be obtained. Wash and filter with anhydrous diethyl ether three times, and dry in vacuum for 8 hours to obtain a white or light yellow solid, dry in vacuum at 80°C for 24 hours.
Embodiment 3
[0036] The preparation of embodiment 3.2-(2-hydroxyethyl)-1-methylpyrazole chloride:
[0037]
[0038] Put 1.0mol of methylpyrazole into a 500mL three-necked flask, stir and drop 1.0mol of chloroethanol at 80°C, and keep it warm for 12 hours after the addition is completed. After the reaction mixture is cooled to room temperature, a white or light yellow jelly can be obtained. The substance was washed with anhydrous ether and filtered three times, and dried in vacuum for 8 hours to obtain a white or light yellow solid, which was dried in vacuum at 80°C for 24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com